Abstract
Introduction
The therapeutic efficacy of allogeneic stem cell transplantation (allo-SCT) relies mostly on the immune-mediated graft-versus-tumor (GVT) activity of the graft. However the beneficial effect of GVT is counterbalanced by the destruction of the tissues of the recipient by the immune effectors of the donor, termed graft-versus-host disease (GVHD). Despite G-CSF mobilized peripheral blood stem cells (PBSC) being used for more than 20 years, literature investigating the impact of the different graft cell subsets on outcome, remains scarce. Therefore, this study aimed to investigate the impact of a comprehensive set of immune cells contained within PBSC grafts on patients' outcome after allo-SCT, using the composite endpoint of GVHD-free and progression-free survival (GPFS) as primary endpoint. Indeed, some cell subset may lead to a decreased incidence of relapse but at the expense of an increased incidence of GVHD or vice versa. The use of the GPFS composite endpoint would allow to overcome this limitation, identifying cellular subsets that correlate with cure without ongoing morbidity.
Patients and methods.
80 consecutive patients who underwent allo-SCT in a single center using G-CSF-mobilized PBSCs between 2010 and 2013 and for whom frozen aliquots of the PBSC grafts were available, were included in this study. Immune cell subset quantification was performed by multi-color flow cytometry. Patients median age was 57 years, 57% of patients had a myeloid malignancy and 43% a lymphoid malignancy. Disease risk index was low or intermediate in 79% of patients and high or very high in 21% of patients. The majority of patients received a reduced-intensity conditioning regimen (81%) and in-vivo T-cell depletion using anti-thymocyte globulin (ATG) (91%). GVHD prophylaxis was cyclosporine alone (34%), or in combination with either mycophenolate mofetil (59%) or methotrexate (7%). 65% of patients received grafts from unrelated donors and 35% from matched related donors.
Naïve and memory T cell and B cell subsets, regulatory T cells, invariant natural killer T-cells (iNKT), natural killer cells (NK) and dendritic cell subsets were analyzed. Cell subsets were selected for the purpose of this study based on the absence of a strong correlation with another graft subset (Pearson or Spearman correlation >0.8). P-values were not adjusted for multiple comparisons, but only covariates for which the q value is <0.2 are presented. GPFS was defined as survival without disease progression, grade III-IV acute GVHD or chronic GVHD requiring systemic therapy.
Results
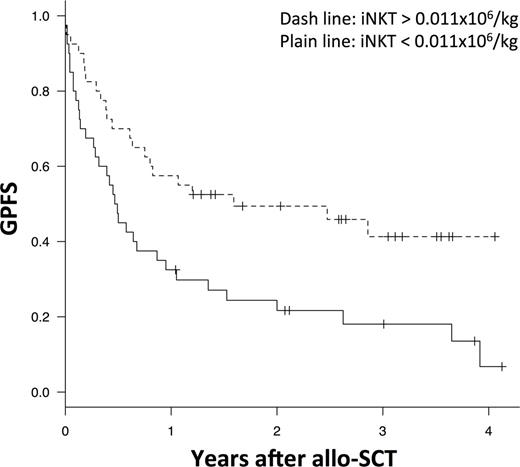
Median follow-up was 36 months. The median numbers of nucleated cells, CD34+ cells, and total T-cells in the allograft were 102.9, 7.2, and 20.7 x 106/kg, respectively. The numbers of nucleated cells, CD34+ cells, T-cells, B-cells, NK cells, and dendritic cells in the PBSC graft, were not significantly associated with overall survival (OS) and GPFS. Invariant NKT cells were the only cell subset associated with GPFS with univariate p-value <0.01 and q values <0.2. Stratifying the entire group of transplant recipients by median value for iNKT cells (median number 0.011x106/kg) showed better GPFS among patients receiving larger number of iNKT cells, being 49% at 2 years, versus 22% for patients receiving <0.011x106/kg iNKT/Kg (p=0.007) (Figure 1). In multivariate analysis, adjusted for patient age, type of donor, disease risk index, GVHD prophylaxis, conditioning regimen intensity and use of ATG, dose of iNKT cells was the only parameter with a significant impact on GPFS (hazard ratio: 0.45, 95% CI: 0.25-0.80, p=0.007). Transplantation with more than the median number of iNKT cells was also associated with a trend toward an improved progression-free survival (59% versus 39% at 2 years, p=0.12) and a decreased risk of extensive chronic GVHD (5.1% versus 17.5% at 2 years, p=0.07). In contrast, the number of iNKT cells transplanted has no impact on grade II-IV aGVHD (10% versus 15%, p=0.49) or overall survival (60% versus 55%, p=0.51).
Conclusion
Among patients undergoing allo-SCT and receiving a PBSC graft, a higher number of iNKT, a regulatory cell population implicated in immune tolerance and tumor surveillance, is associated with an improved GPFS. These data may pave the way for prospective and active interventions aiming to manipulate the graft content in order to improve allo-SCT outcome.
Moreau:Millennium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees. Mohty:Janssen: Honoraria; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal