Abstract
Background
Entospletinib (GS-9973) selectively inhibits spleen tyrosine kinase (SYK), a critical signaling component of the BCR pathway that is expressed primarily in cells of hematopoietic lineage including normal and malignant B-lymphocytes. Entospletinib is currently in phase II clinical trials, where it has demonstrated both a high degree of safety as well as efficacy against chronic lymphocytic leukemia (Sharman, J., et al. Blood, 2015) and other B cell malignancies. Despite these successes, new therapeutic options, including combinations with standard of care agents, are needed in order to achieve the goal of curing disease through finite treatment. We show here that the combination of entospletinib and vincristine causes synergistic apoptosis in vitro in a broad panel of cell lines derived from hematological cancers including diffuse large B cell lymphoma (DLBCL), acute lymphocytic leukemia, follicular lymphom), multiple myeloma, and acute myelogenous leukemia. We also evaluated and compared the in vivo efficacy of entospletinib and vincristine as singe agents and in combination in a DLBCL tumor xenograft model using the SU-DHL-10 cell line.
Methods
In vitro growth inhibition of a panel of malignant hematological cell lines was assessed using CellTiter-Glo™ Assay (Promega) after 72h incubation with entospletinib or vincristine alone or in combination. Synergy was evaluated using the Bliss model of independence (Meletiadis, J., et al., Med Mycol, 2005). In vivo, SU-DHL-10 cells (5 x 106 cells) were implanted subcutaneously in the axilla in male SCID beige mice. All mice were sorted into study groups on Day 16 such that each group's mean tumor volume fell within 10% of the overall mean (197mm3). Dosing was initiated on Day 16 and animals were dosed for 17 days. Plasma concentrations of entospletinib and vincristine were assessed on Day 19, and the entospletinib 75 mg/kg dose was lowered on Day 22 to 50 mg/kg to approximate the human achievable SYK target coverage of EC80. Efficacy and tolerability were evaluated by tumor measurements and body weight monitored three times weekly. Tumor burden data were analyzed by the application of a two-way analysis of variance (ANOVA), with post-hoc analysis.
Results
In vitro combinations of entospletinib with low concentrations of vincristine resulted in marked inhibition of cell proliferation and induction of apoptosis in a broad panel of 19 tumor cell lines representing major B cell malignancies including DLBCL. The combination of entospletinib with vincristine had a profound inhibitory effect on proliferation in all subtypes of DLBCL. Entospletinib was evaluated at a concentration equivalent to the Cminof the clinical dose and vincristine was used at concentrations (≤ 10 nM) that had little to no significant single agent effect in these cell lines.
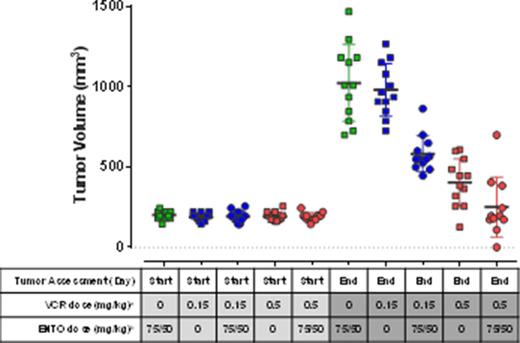
In vivo in a SU-DHL-10 xenograft model, entospletinib dosed alone at 25 or 75/50 mg/kg significantly inhibited tumor growth, causing 39% and 20% tumor growth inhibition (TGI), respectively, compared to the vehicle-treated control group. Vincristine administered at either 0.15 and 0.5 mg/kg Q7D x 3 also resulted in significant TGI (42% and 85% TGI, respectively). The addition of entospletinib (75/50 mg/kg) to 0.5 mg/kg or 0.15 mg/kg vincristine resulted in a significant increase in TGI from 85% to 96% (p= 0.001) and 42% to 71% (p< 0.0001), respectively. The addition of entospletinib (25 mg/kg) to vincristine did not significantly increase the tumor growth inhibition. While the groups receiving either entospletinib or vincristine as single agents had no complete or partial tumor regression, 50% of the mice receiving the combination of 75/50 mg/kg entospletinib with 0.5 mg/kg vincristine had partial responses, 8% had complete regression and 8% were tumor free at the end of study (Figure 1).
Conclusion
Entospletinib and vincristine demonstrated efficacy and tolerability both alone and in combination in the SU-DHL-10 DLBCL cell line xenograft model in SCID beige mice. Vincristine combinations with entospletinib showed significantly greater efficacy than vincristine alone. These data support the further clinical development of entospletinib in combination with vincristine for the treatment of DLBCL.
a ENTO: PO: Q12H x 2 (Day 16-32)
b VCR: IV: Q7D x 3 (Days 18, 25, 32)
Tumor Regressions in an Entospletinib/ Vincristine Treated Murine DLBCL Xenograft
Tumor Regressions in an Entospletinib/ Vincristine Treated Murine DLBCL Xenograft
Axelrod:Gilead Sciences: Employment, Equity Ownership. Fowles:Gilead Sciences: Employment, Equity Ownership. Silverman:Gilead Sciences: Employment, Equity Ownership. Clarke:Gilead Sciences: Employment, Equity Ownership. Tang:Gilead Sciences: Employment, Equity Ownership. Rousseau:Gilead Sciences: Employment, Equity Ownership. Webb:Gilead Sciences: Employment, Equity Ownership. Di Paolo:Gilead Sciences: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal