Abstract
Global phase I/III pharmacokinetic and efficacy study comparing CT-P10, a biosimilar candidate to the rituximab reference product in patients with advanced stage follicular lymphoma (AFL)
CT-P10 is a biosimilar candidate to the reference rituximab product, EU-approved MabThera® and US-licensed Rituxan®. CT-P10 has an identical amino acid sequence and highly similar physicochemical and in vitro functional properties to its reference drug. In patients with rheumatoid arthritis (RA), CT-P10 has demonstrated compelling similarity in pharmacokinetics, pharmacodynamics, efficacy, safety and immunogenicity (http://acrannualmeeting.org/abstracts/abstract-archives/,#1736 in 2013 and #1508 in 2014).
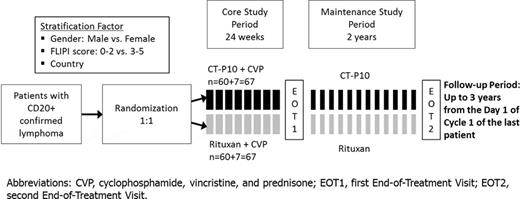
This study is a randomized, parallel-group, active-controlled, double-blind, phase I/III study designed to demonstrate similarity in pharmacokinetics and non-inferiority in efficacy of CT-P10 to rituximab when co-administered with cyclophosphamide, vincristine, and prednisone (CVP) (NCT02162771). In this international multicenter trial, 134 patients with untreated advanced stage follicular lymphoma (AFL) will be administered CT-P10 or rituximab for 24 weeks at 3 weeks intervals, defined as Core Study Period. Patients who at least achieve a partial response after the Core Study Period will continue treatment with CT-P10 or rituximab as maintenance therapy over 24 months in 2 monthly intervals (Figure 1).
The patients ≥18 years, with a histologically confirmed FL of grade 1 to 3a, Ann Arbor Stage III or IV disease, at least 1 measurable tumour mass that has not previously been irradiated, confirmed CD20+ lymphoma, ECOG performance status of 0-2, and adequate bone marrow, hepatic, and renal function reserves are included.
The study has been initiated at >110 clinical sites across Europe, Latin America, Asia and Africa. Currently 83 patients were enrolled in this study, and a blinded initial review of initial safety data was carried out by the Data Safety Monitoring Board. As expected from the highly similar profile of CT-P10 accomplished through analytical studies and the CT-P10 RA trial (NCT01534884, NCT01873443), preliminary safety findings in terms of adverse events (AEs), serious AEs, AEs of special interest and survival characteristics with CT-P10 in AFL patients mirror those with the historical data of rituximab reference product. As currently 4 SAEs related to a study drug (CT-P10/Rituximab) were reported and these were resolved without sequelae. Qualitative feedback from study investigators throughout all regions indicates overall positive experience. Taken together, the study provides AFL patients with early access to a biosimilar investigational product assuring improved standards of care and long-term survival outcomes.
In conclusion, it is expected that this ongoing study will demonstrate that CT-P10 is well tolerated in AFL patients with a similar safety profile to the rituximab reference product.
Study Design
Kim:CELLTRION, Inc.: Consultancy, Honoraria. Ogura:CELLTRION, Inc.: Consultancy, Honoraria. Buske:CELLTRION, Inc.: Consultancy, Honoraria. Coiffier:CELLTRION, Inc.: Consultancy, Honoraria. Sancho:CELLTRION, Inc.: Research Funding. Jurczak:CELLTRION, Inc,: Research Funding. Kim:CELLTRION, Inc,: Research Funding. Nagarkar:CELLTRION, Inc,: Research Funding. Zhavrid:CELLTRION, Inc,: Research Funding. Hernandez Rivas:CELLTRION, Inc.: Research Funding. Vinnyk:CELLTRION, Inc.: Research Funding. Tsirigotis:CELLTRION, Inc.: Research Funding. Viero:CELLTRION, Inc.: Research Funding. Volodicheva:CELLTRION, Inc.: Research Funding. Fourie:CELLTRION, Inc.: Research Funding. Soler:CELLTRION, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal