Abstract
BACKGROUND
The incidence of non Hodgkin's lymphoma (NHL) increases with age, over one third of NHL cases involves elderly patients >70 years of age.
As a matter of fact, many elderly patients (pts) are not enrolled in controlled studies because they do not meet the inclusion criteria. This is the reason why the data from trials on elderly cases are not representative for the whole elderly population. Consequently, many of these patients do not benefit of new therapeutic progresses and the treatment is not yet adequate.
Moreover in case of relapse, salvage therapy in the elderly is virtually absent, and the prognosis is extremely poor.
Lenalidomide is an immunomodulatory drug with anti-angiogenetic and anti-neoplastic action on cancer cells and also has other anti-tumor activity by acting on the neoplastic microenvironment. Both monotherapy and combination of Lenalidomide with rituximab have shown efficacy in terms of overall response rate (ORR, Overall Response Rate) in the setting of salvage therapy in relapsed/refractory DLBCL, with an acceptable rate of hematologic and extra-hematological toxicities
PATIENTS AND METHODS
In the period 2013-2014 we consecutively treated 12 elderly patients affected by advanced DLBCL relapsed/refractory.
Median age at the start of treatment was 79 years (yrs) (62-86), 5 out 12 was over 80 yrs and 4 out 12 over 75 yrs. The median number of previous treatment was 3 (2-4), 3 pts were transplanted and 4 patients was refractory to previous line of therapy.
The treatment scheme included at first cycle: Rituximab 375 mg/m2 i.v. days 1, 8, 15, 22, Desametasone 5 mg p.o. days 1, 8, 15, 22 and Lenalidomide 15 mg/die p.o. from day 2 to 22. From the second to the sixth cycle we administered Rituximab 375 mg/m2 i.v. days 1, 14, Lenalidomide 20 mg/die p.o. from day 2 to 22
RESULTS
The overall response rate has been 75% (CR,PR,SD), 3 patients out 12 (25%) achieved a CR and 3 PR (25%).
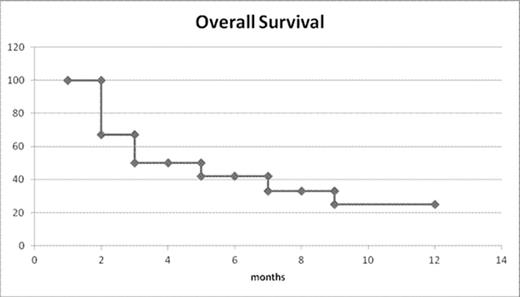
The median PFS was 6 months and with a median follow up of 1 year the overall survival is 25%.(fig.1)
All deaths are due to lymphoma progression and the 3 patients in CR are still alive in CCR.
CONCLUSIONS
In this elderly and heavily pretreated group of patients our scheme containing Lenalidomide-Rituximab has shown an high rate of response with a good rate of CR and a promising trend in overall survival.
The treatment appears feasible and safe also in this particular group of frail patients and even if we need more data to confirm we think it will be possible to extend this therapy in frail elderly patients in a most precocious line of treatment.
Offidani:Celgene, Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal