Abstract
Introduction: Anthracyclines plays a key role in the treatment of Diffuse Large B cell Lymphoma (DLBCL). There is a great concern about its cardiotoxicity, especially among elderly patients with raising age and related morbidities. Liposomal doxorubicin (LD) has proven to be efficacious and less cardiotoxic in combinations of neo-adjuvant chemotherapy regimes through phase II and III studies in breast cancer. There are some phase II studies in DLBCL but to our knowledge, controlled randomized studies are pending.
Aim: To analyze the efficacy and toxicity of LD (Myocet¨) in DLBCL patients with cardiovascular/cardiopathy risk, in comparison with a control population who received the standard therapy during the same period of time.
Methods: We conducted a retrospective cohort study in patients with de novo DLBCL treated with standard immunochemotherapy, comparing conventional Doxorubicin vs LD. Selection of a 3.3 vs 1 ratio was used to increase statistic power. Criteria to indicate LD therapy were any of the following: left heart ejection fraction < 50%, cardiovascular risk factors or prior history of cardiopathy. Overall response (OR), complete/uncertain response (CR/CRu), progression free survival (PFS) and overall survivall (OS) were analyzed. Toxicity was evaluated with CTCAE v.4.0. and the used statistical package was SPSS v18.0.
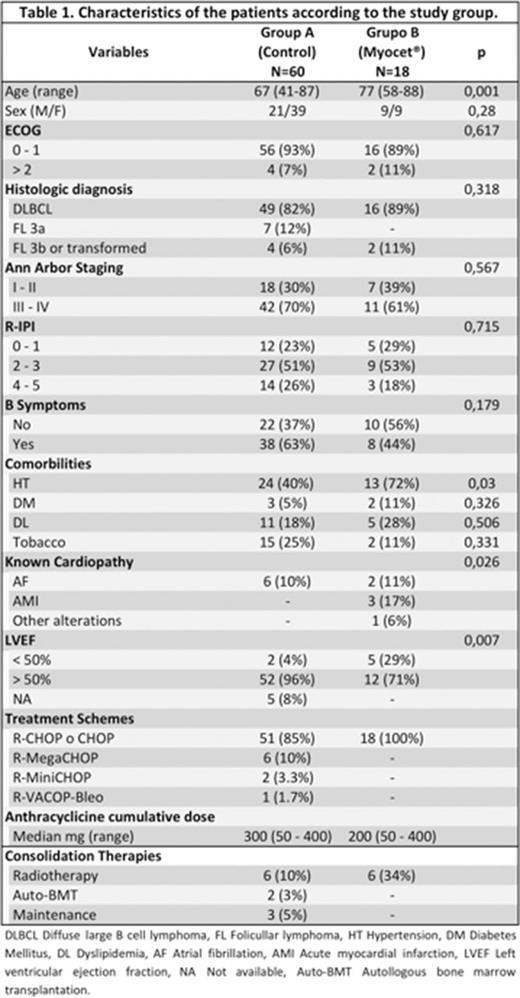
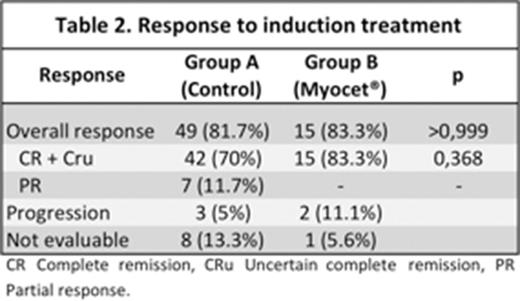
Results: 78 patients (pts) treated between March 2006 and June 2012 were selected: 60 pts in the control group (group A) and 18 in the study arm (group B). Their characteristics are shown in table 1. Homogeneity was observed, except in LVEF < 50%, older age and hypertension in group B. Efficacy: 69 pts were evaluable for response, with similar OR rate and CR+CRu in both groups (see table 2). Response was consolidated in 17 pts: 22% in group A and 40% in group B. With a median follow up of 4.3 years for surviving pts (0.74 yrs - 8 yrs), actuarial OS and PFS for the whole series were 64% ±SD 13% and 55% ±SD 7%, respectively, without significant differences between both arms (OS: 64% vs 77%; p=0,7 and 56% vs 55%; p=0,9). Toxicity: Hematological toxicity was 43% vs 11%, grade 3 neutropenia 43% vs 6% and febrile neutropenia 37% vs 11%, all significantly different and favoring group B. These differences were probably due to the systematical use of Peg- GSF in group B. Four (5%) cardiac events were observed in group B: 1 heart failure and 3 arrhythmias. Currently, 16 (21%) pts have died, 11 due to lymphoma, 4 due to infection grade 5 during induction treatment and 1 suicide.
Conclusion: In this study, the association of LD to immunochemotherapy showed the same efficacy as conventional Doxorubicin in fragile patients, without increased toxicity.
Acknowledgment:We are grateful to TEVA for their contribution with the CRO for the statistical analysis.
Alegre:Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal