Abstract
Background
Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA) is the only viral protein consistently expressed by EBV. Anti-EBNA-1 antibodies (EBNA-Ab) status has long been the method to diagnose the latent EBV infection to date. Both positivity of EBV-encoded small RNA in situ hybridization using lymph node and positivity of EBV-DNA using peripheral blood meant that poor therapeutic outcomes in patients with peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) and with NK/T-cell lymphoma, respectively. It has been unknown whether EBNA-Ab status has another clinical implication except knowing latent EBV infection.
Methods
This is an observational trial in single cancer institute. EBNA-Ab has routinely measured before the treatment in patients with PTCL-NOS, who were diagnosed or treated in our hospital from July 2001 to December 2014. Then, we analyzed that whether these patients attributed their therapeutic outcomes to the each value of their pretreatment EBNA-Ab titer. Primary objective was to evaluate a prognostic value of EBNA-Ab titer for one-year overall survival (OS). Secondary objective was response rate.
Results
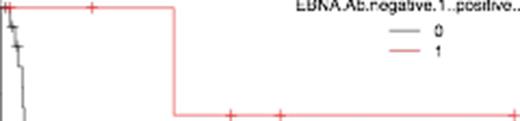
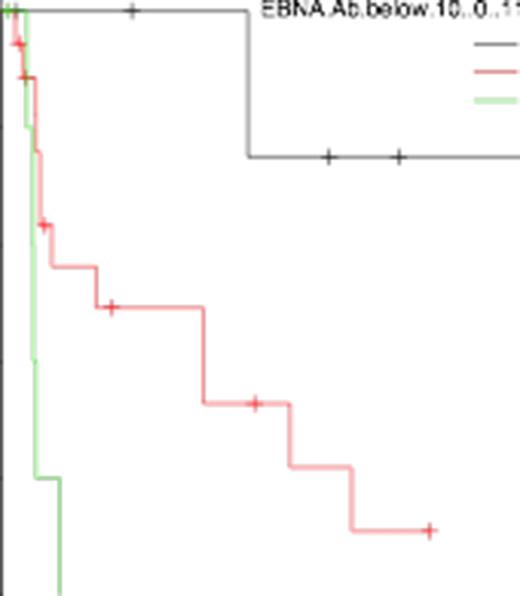
In total of 30 cases, 24 showed EBNA-Ab positive (titer ≥ 10). Baseline patients characteristics run as follows; median age was 63 (26-83), 22 were men, 18 were Ann Arbor stage ≥ 3, 11 were IPI ≥ 3, 9 showed elevated LDH. All patients were given six cycles of CHOP except one patient (CHOEP). Overall response rate (ORR) was 40% and complete response rate (CRR) was 27%. 40% showed progression disease. The median OS was 51.4 months. At the median follow-up of 12 months, the pretreatment EBNA-Ab level demonstrated significant correlation with prognosis. OS was 52.7% (95% confidential index [CI]: 30-71) and 100% (95%CI: 100-100) in cases that EBNA-Ab positive and EBNA-Ab negative, respectively (Figure 1, p value of log-rank test = 0.013). Furthermore, we compared outcomes in the three groups: EBNA-Ab < 10, 10 ≤ EBNA-Ab ≤ 60, and EBNA-Ab > 60. Each group has same proportion of age > 60 (p value of fisher exact test = 0.26), sex (p = 0.51), IPI ≥ 3 (p = 0.85), stage ≥ 3 (p = 0.09), and elevated LDH (p = 0.64). ORR was 33% vs. 44% vs. 33% and CRR was 33% vs. 28% vs. 17% in cases that EBNA-Ab < 10, 10 ≤ EBNA-Ab ≤ 60, and EBNA-Ab > 60. In terms of OS, 100%, 63%, and 20% in cases that EBNA-Ab < 10, 10 ≤ EBNA-Ab ≤ 60, and EBNA-Ab > 60, respectively (Figure 2, p value of log-rank test = 0.0007).
Conclusion
As these results demonstrated, in this study, patients with high-level titer of EBNA-Ab demonstrated shorter OS. Especially, EBNA-Ab titer > 60 cases showed the worst outcome. By contrast, EBNA-Ab negativity demonstrated significantly longer OS. Higher EBNA-Ab might be an independent marker that associated with poorer outcomes in patients with PTCL-NOS.
OS between EBNA-Ab < 10, 10 ≤ EBNA-Ab ≤ 60, and EBNA-Ab > 60 arms (p = 0.0007)
Mishima:Chugai Pharmaceutical CO., LTD.: Consultancy. Nishimura:Chugai Pharmaceutical CO., LTD.: Consultancy. Yokoyama:Chugai Pharmaceutical CO., LTD.: Consultancy. Hatake:Chugai Pharmaceutical CO., LTD.: Other: lecture speaking.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal