Abstract
Background: Classical Hodgkin lymphoma (cHL) is the most common lymphoid malignancy under the age of 30. Despite the relatively high curability, about 20-30% of patients relapse after front-line therapy. Since the International Prognostic Factors Project score became obsolete in the era of dose-intensive regimens (BEACOPP), a clinically applicable tool for outcome prediction is lacking. Recent years have brought much novel information about close Hodgkin/Reed-Sternberg (HRS) microenvironment cross-talk promoting tumor growth. Many studies have tested the predictive value of microenvironment cells using immunohistochemistry but none have been concerned with HRS cell density.
Aim: To assess the prognostic role of HRS cell density in lymph node biopsies using a novel automated system for scanning large tumor sample areas in cHL patients.
Methods: Thirty-eight high-quality tissue samples obtained at time of cHL diagnosis were analyzed. The median age at diagnosis was 35 (17.5-93) years; the male-to-female ratio was 0.66:1. The lymphoma subtypes were nodular sclerosis (NS) in 25 (66%) and mixed cellularity (MC) in 13 (34%) patients. At the time of analysis, detailed baseline clinical information was available in 35 (92%) patients. Ann Arbor stages I through IV were observed in 2, 18, 9 and 6 patients, respectively. Systemic symptoms were present in 26 (75%) cases and large lymphoma mass (≥5 cm) was detected in 19 (56%) patients. German Hodgkin Study Group stages were: limited in 4 (12%), intermediate in 8 (23%) and advanced in 23 (66%) patients. Chemotherapy was given to 35 patients (92%): BEACOPP in 20 (57.1%), ABVD in 6 (17.1%), Stanford V in 4 (11.4%) and other (COPP, COPP/ABV) in 5 (14.3%) cases. Involved-field radiotherapy was applied in 11 (29%) cases. Tissue array analyses were performed using the TissueFAXS (TissueGnostics, Austria) system combining detailed morphologic information offered by microscopy with the scientific accuracy of multi-channel flow cytometry. Data were analyzed with TissueQuest software. Paraffin-embedded biopsies were prepared from pre-selected diagnostic samples stained with hematoxylin-eosin for morphology analyses and using CD30 (HRS) for cell density analyses. Each sample was previewed by the scanning software and only significant tumor tissue was manually gated by an experienced pathologist for further analyses. Fibrotic (≥10%), necrotic or residual lymphatic structures were excluded.
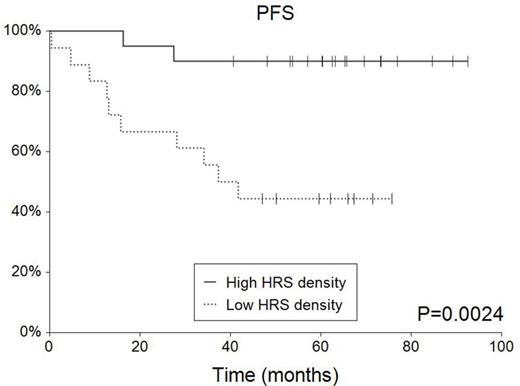
Results: After treatment, 31 (82%) patients achieved complete remission, three partial remission, one stable disease and three patients progressed. After a median follow-up of 64.3 months, 9 (24%) patients relapsed or progressed and 7 (18%) of them died. Five-year overall survival (5-y OS) reached 81.0% (95%CI 0.68-0.94); 5-year progression survival (PFS) was 68.3% (95%CI 0.53-0.83). Tissue data: The mean scanned area covered 27.2±13.5 mm2 with the mean (median) total number of cells 461,504±286,491 (466,138). The mean (median) total cell density and CD30+ HRS cell density were 17,886±7,884 (16,557) and 279±232 (181) cells per mm2, respectively. Total cell density was 1.28-fold higher (p=0.14) and HRS cell density 1.44-fold (p=0.19) higher in MC compared to NS. HRS cell density did not correlate with sex (p=0.46), systemic symptoms (p=0.38), tumor bulk (p=0.34) or Ann Arbor stage (p=0.59). There was a trend for older age among low HRS cell density (below the median) patients (p=0.19). Low HRS cell density was associated with inferior 5-y PFS (44.4% vs 90.0%, p=0.0024) and OS (57.5% vs 100%, p=0.002). Multivariate Cox regression identified low HRS cell density as an unfavorable prognostic factor for PFS (p=0.037, HR=5.50) independent of age and lymphoma subtype.
Conclusions: This is the first evidence about the possible predictive role of HRS cell density in cHL patients. Low density at diagnosis was associated with 5.5-fold higher risk of lymphoma progression or death. Automated image analysis is a new tool overcoming technical limitations caused by small (microarray) samples in lymphomas with high intra-tumor heterogeneity. Further analyses will define the role of HRS cell analysis in cHL in the context of risk-adapted and CD30-targeted therapies.
Acknowledgment - Supported by: Palacky University Olomouc (IGA-LF-2015-001), Takeda Restricted Educational Grant and J. W. Fulbright Commission.
Prochazka:Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal