Abstract
Advances in treatment over the last decades have resulted in almost 90% 5-year survival of children with acute lymphoblastic leukemia (ALL). Nonetheless, a minor group with unfavorable prognosis becomes candidates for hematopoietic stem cell transplantation (HSCT). After HSCT, blast count, minimal residual disease (MRD) and chimerism analysis are used as markers for successful engraftment and risk of relapse. However, additional markers are needed to complement the existing ones, as 30 % of ALL-patients still experience relapse after HSCT. Mutations in the tumor suppressor gene TP53 are detected in 2% at diagnosis of pediatric ALLs, but in 11-28 % after relapse. The aim of this study was to evaluate if alterations in the expressions of the TP53 encoded protein p53, analyzed by immunohistochemistry, could act as a prognostic marker in pediatric ALL patients post HSCT.
Paraffin-embedded bone marrow samples were collected retrospectively from 49 children diagnosed with pre-B ALL and, treated with HSCT at Karolinska University Hospital between 1997-2010. Patients samples were requested from six different times during the course of the disease; time of diagnosis, before HSCT and at routine controls after HSCT at 0-3, >3-6, >6-12 and >12-24 months. For no patient data was available from all time periods. In total 176 samples were collected. A control group consisted of fifty-five children, where a bone marrow biopsy had been performed due to suspected bone marrow disorder, but a malignant blood disease had been excluded.
Tissue micro array (TMA) prepared microscope slides were stained for the p53 protein using a monoclonal mouse anti-human antibody (clone DO-7, Leica), which detects both the wild-type and mutant forms of p53. Protein expression was examined by two independent examiners in a light microscope at high magnification (40X). Cells positive for the p53-protein identified by brown nuclear staining, were counted and calculated as a percentage of total cells per sample for each antibody respectively. Mean number of cells counted per sample was 695. Some samples were lost prior to final analysis due to either; sample core lost during TMA (n=12) or, sample had <100 cells which was minimum number of cells for inclusion in analysis (n=10). The final analysis included 154 bone marrow samples from 49 patients and 55 control samples.
Data was analyzed in Statsoft Statistica 12.7. Nonlinear logistic regression was used to evaluate predictive value of the protein. Data was analyzed independently at each of the following times; diagnosis, time of HSCT and at 0-3, >3-6, >6-12 and >12-24 months post HSCT. A p value < 0.05 was considered significant.
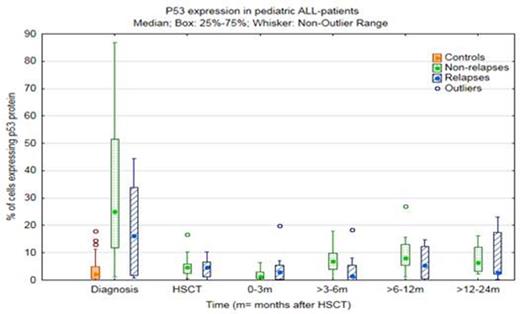
Interestingly, we found that a low percentage of cells positive for p53 protein expression in the bone marrow at >3-6 months after HSCT was associated with increased risk of relapse in pediatric ALL patients, odds ratio 0.82 (confidence interval 0.68-0.99) p= 0.041. Figure I shows the range of p53 expression in the two groups individually at each time.
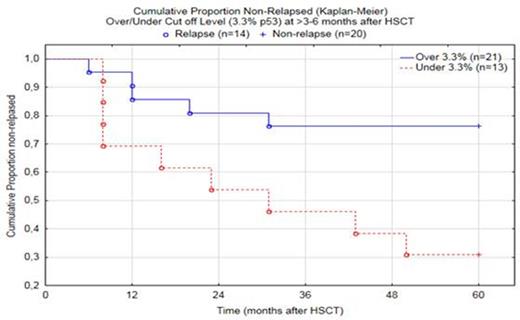
At >3-6 months after HSCT, the cut off value was calculated to 3.3% p53 positive cells, and was correct for 16 of 20 non-relapse cases and for 9 of 14 relapse cases (overall 73.5% correct). All relapses occurred between 6-50 months after HSCT (mean=20, median=14).
The lower amount of p53 positive cells in the relapsed patients compared to the non-relapsed patients could possibly be due to an underlying mutation in TP53 that has altered the ability of producing a protein. This would be in similarity with the literature where it is suggested that TP53 mutations are more common at relapse in pediatric ALL patients.
In summary, a lower expression of p53 protein in the bone marrow at >3-6 months after HSCT was significantly associated with increased risk of relapse. Evaluation of p53 protein expression by immunohistochemistry, in bone marrow from pediatric ALL-patients that undergo HSCT may be a potential additional marker for predicting relapse.
Range and median percentage of p53 positive cells at different times in bone marrow from children with pre-B ALL. At >3-6 months after HSCT a higher quantity of p53 positive cells indicated a relapse free prognosis.
Range and median percentage of p53 positive cells at different times in bone marrow from children with pre-B ALL. At >3-6 months after HSCT a higher quantity of p53 positive cells indicated a relapse free prognosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal