Abstract
INTRODUCTION The Bcl-2 inhibitor venetoclax (GDC-0199/ABT-199) has yielded promising results in patients with relapsed/refractory chronic lymphocytic leukemia (CLL), both as monotherapy and in combination with rituximab. The CLL11 trial demonstrated that combining obinutuzumab, a type 2, glycoengineered anti-CD20-antibody, with chlorambucil resulted in improved overall survival compared to chlorambucil alone in patients with previously untreated CLL and coexisting medical conditions. Prior to opening the randomized phase of the current CLL14 trial, the successor trial to CLL11, a safety run-in phase of previously untreated patients with CLL and coexisting medical conditions was performed to assess the tolerability of obinutuzumab and venetoclax. Here, we report preliminary results of this safety run-in phase after completion of recruitment.
METHODS The protocol specified to enroll 12 previously untreated patients with confirmed CLL and with coexisting medical conditions assessed by cumulative illness rating scale (CIRS) total score > 6 and/or estimated creatinine clearance (CrCl) < 70 mL/min requiring treatment (according to NCI/iwCLL criteria into the safety run-in phase of the CLL14 trial. All patients received 6 cycles of obinutuzumab and venetoclax followed by 6 additional cycles of venetoclax. Obinutuzumab was administered intravenously with 100 mg on day 1, 900 mg on day 2 (option to deliver 1000 mg on day 1), 1000 mg on day 8 and day 15 of cycle 1 and 1000 mg on day1 for cycles 2-6. A gradual weekly dose ramp-up of venetoclax with 20 mg, 50 mg, 100 mg, 200 mg up to 400 mg was administered starting at day 22 of cycle 1. Risk assessment for tumor lysis syndrome (TLS) based on absolute lymphocyte count and the largest diameter of measurable lymph nodes was performed before treatment in order to direct prophylactic measures. Study defined stopping criteria for all 12 patients included: one treatment-related death or one grade 4 adverse event related to a clinical TLS despite protocol-specified prophylaxis. Adverse events were graded per the NCI CTCAE v.4 criteria.
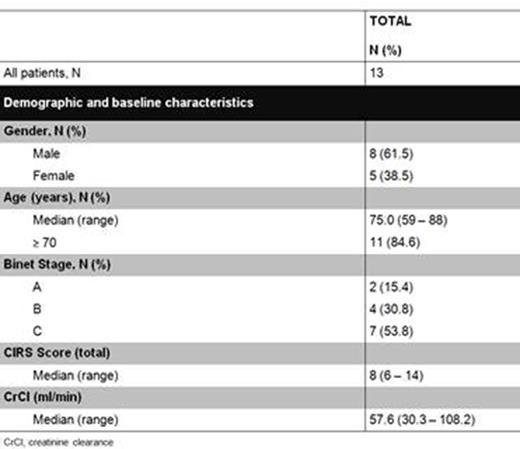
RESULTS Between December 2014 and April 2015, 13 previously untreated patients from Australia, Canada, Germany, New Zealand, United States and Spain were enrolled; baseline patient characteristics are summarized in Figure 1. The median age was 75 years (range 59 - 88) and 54% of the patients were classified as Binet stage C; six patients were assessed medium risk and 7 patients high risk for TLS. At the timepoint of data cut-off, 12 of 13 patients have been on treatment for at least 4 weeks and completed the venetoclax dose ramp up. The median time on treatment with venetoclax is 64.5 days (range 34-153 days). One patient developed a grade 4 infusion related reaction (IRR) during the first dose of obinutuzumab and was therefore withdrawn from the trial according to the protocol requirements. All patients experienced at least 1 adverse event. Adverse events are summarized in Figure 2. Of note, 1 patient developed a self-limited grade 4 elevation of transaminases secondary to obinutuzumab. No clinical TLS was reported. Two patients developed laboratory TLS. One patient was classified as medium risk for TLS and developed hyperkalemia and hyperuricemia after the 100 mg infusion of obinutuzumab on day 1 of cycle 1. The second patient was classified as high risk for TLS (including a lymph node measuring 110 mm in diameter) and developed hypocalcemia, hyperphosphatemia and hyperuricemia after receiving 200 mg of venetoclax on day 15 of cycle 2. Neither event resulted in interruption or dose modification of study treatment. Rapid reduction in the peripheral lymphocyte count was observed in all 12 patients treated with the combination regimen. Initial response data is anticipated for all 12 patients in the next 6 months per the protocol schedule.
CONCLUSIONS The treatment regimen developed for the experimental arm of the CLL14 trial comprising obinutuzumab monotherapy for one cycle followed by venetoclax and obinutuzumab in previously untreated, elderly patients with CLL and coexisting medical conditions appears tolerable. The majority of enrolled patients were older than 70 years of age and many of them had clinically meaningful comorbidities in addition to CLL. None of the protocol defined stopping criteria for the safety run-in phase of the study were met. The randomized phase of the CLL14 trial was opened in August 2015.
Fischer:Roche: Other: Travel Grants. Off Label Use: GAZYVA (obinutuzumab) is a CD20-directed cytolytic antibody and is indicated, in combination with chlorambucil, for the treatment of patients with previously untreated chronic lymphocytic leukemia (CLL). This abstract reports on obinutuzumab in combination with venetoclax for previously untreated CLL. Venetoclax is an investigational drug that is not yet approved in this indication. Fink:Roche: Honoraria, Other: travel grant. Bishop:Roche: Employment. Dixon:Roche: Employment, Equity Ownership. Choi:Gilead: Consultancy, Other: Advisory Board, Speakers Bureau; AbbVie: Consultancy, Other: Advisory Board, Research Funding. Weinkove:Avalia Immunotherapies: Consultancy; Janssen New Zealand: Consultancy; Roche New Zealand: Other: Travel support for conference. Robinson:Lundbeck: Other: Advisory boards; Novartis: Other: Advisory boards; CSL: Other: Advisory boards; Bering: Other: Advisory boards; Roche: Honoraria, Other: Advisory boards; Bayer: Other: Advisory boards; Pfizer: Other: Advisory boards; Celgene: Other: Advisory boards; Biogen Idec: Other: Advisory boards; Gilead: Other: Advisory boards; Janssen: Other: Advisory boards; Baxter: Other: Advisory boards. Dreyling:Roche: Honoraria, Research Funding. Opat:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Subsidised drug. Owen:Gilead: Honoraria; Lundbeck: Honoraria; Janssen: Honoraria; Roche: Honoraria. Tausch:Gilead: Other: Travel support. Ritgen:Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding. Humerickhouse:AbbVie: Employment, Equity Ownership. Humphrey:Roche: Employment. Wenger:Genentech, Inc.: Employment. Goede:Bristol-Myers Squibb: Honoraria; Mundipharma: Honoraria; GSK: Honoraria; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding. Eichhorst:AbbVie: Consultancy; Roche: Consultancy, Research Funding, Speakers Bureau; MundiPharma: Consultancy, Research Funding, Speakers Bureau. Wendtner:AbbVie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Research Funding. Stilgenbauer:Roche: Honoraria, Research Funding. Kipps:Pharmacyclics: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Gilead: Honoraria, Speakers Bureau. Hallek:Mundipharma: Honoraria, Other: Speakers Bureau and/or Advisory Board, Research Funding; Celgene: Honoraria, Other: Speakers Bureau and/or Advisory Board, Research Funding; Roche: Honoraria, Other: Speakers Bureau and/or Advisory Board, Research Funding; AbbVie: Honoraria, Other: Speakers Bureau and/or Advisory Board, Research Funding; Boehringher Ingelheim: Honoraria, Other: Speakers Bureau and/or Advisory Board; Pharmacyclics: Honoraria, Other: Speakers Bureau and/or Advisory Board, Research Funding; Janssen: Honoraria, Other: Speakers Bureau and/or Advisory Board, Research Funding; Gilead: Honoraria, Other: Speakers Bureau and/or Advisory Board, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal