Abstract
Background
Rearrangements of the mixed lineage leukemia (MLL) gene at 11q23 (due to translocation, partial tandem duplication or gene amplification) are frequent in AML. Translocations give rise to fusion genes with AF9, AF6, MLLT10 and ENL, among others; partial tandem duplications or self-fusion fuses the 5' region of an MLL into the other MLL gene; and MLL amplification results in several copies of the wild-type gene.
In this work we report the molecular analysis, gene expression profiling and clinical outcome for a cohort of MLL- rearrangedAML.
Patients and Methods
Herein we include 20 MLL-rearrangement harboring patients, diagnosed in our institution of AML.
MLL gene fusion was detected by cytogenetics; FISH with LSI MLL dual color, Break Apart probe, and chromosome painting probes; 10 cases could be verified by reverse transcription PCR (RT-PCR) and sequencing. Gene expression was analyzed in ten cases by real-time PCR in a reaction with specific primers and SybrGreen. Relative quantification was performed by 2-DDCt method.
Survival analysis was carried-out by Kaplan-Meyer method. Overall survival was considered as the time from diagnostics to exitus.
Results
In a time course from March 2004 to June 2015, the systematic screening for genetic rearrangements in AML patients at diagnosis, revealed 20 patients harboring a rearranged MLL. Fourteen of these were translocations, and in nine of them we could demonstrate the genetic fusion at the mRNA sequence level. There were three amplifications of the MLL gene in double minute (dmin), homogeneously stained region (hsr) and in several marker chromosomes.
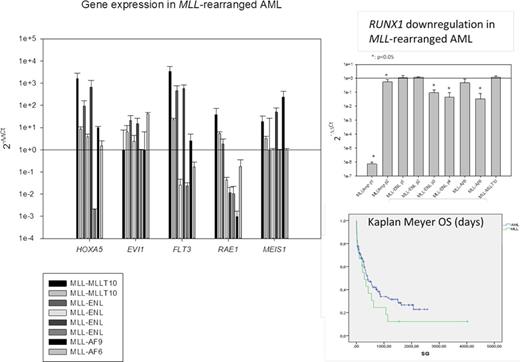
MLL rearrangement harboring patients had a median overall survival of 284±119 (N=18) days and only 25% of them survive at 1.7 years.
Gene expression profiling of AML relevant genes (HOXA5, FLT3, EVI1, MEIS1 and RUNX1) showed a general upregulation of HOXA5, and a heterogenic regulation of FLT3. EVI1 and MEIS1 expression, undetectable in health control's peripheral blood, were upregulated only in some cases, interestingly the only two survivors in the series had undetectable EVI1 expression (Fig 1A). RUNX1 had a downregulation in MLL amplification cases, especially patient with 15-20 MLL copies in HSR in hyperdiploid cells that showed 7.4*107±2.5*107 fold downregulation (p<0.01), almost undetectable by real-time PCR (Fig 1B). Also was significatively (p<0.05) downregulated in two of the four MLL-ENL harboring patients and the only included MLL-AF6 harboring patient.
Conclusions
In this work we show that MLL-rearranged AML have a poor outcome, and most of them should not be included in intermediate-risk karyotype. Accordingly, the classification of MLL cases by the genetic partner or gene expression profile may provide a more precise prognostics. In this sense, overexpression of EVI1 has been previously associated to a worst outcome, but there are other genes that could be useful in prognostics such as MEIS1.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal