Abstract
Introduction
FLT3 is a receptor tyrosine kinase that plays a role in hematopoietic stem/progenitor cell proliferation and survival and is frequently found to be mutated in patients with acute myeloid leukemia (AML). Mutations that lead to constitutive activation of FLT3 (such as internal tandem duplications of the juxtamembrane domain or point mutations involving the kinase domain) are associated with a poor prognosis. This poor prognosis is in part due to an increased relapse rate after allogeneic hematopoietic cell transplant (HCT). Several compounds that inhibit the activity of FLT3 in vitro, including ASP2215, midostaurin and quizartinib, are now being studied in clinical trials for the treatment of AML. Inhibition of the formation of phospho-FLT3 has been correlated with clinical anti-leukemia effects and remissions.
Methods
In this pilot study we use immunohistochemistry to measure the levels of activated FLT3 in bone marrow biopsies of patients prior to and during treatment inclinical trials at our institution to determine the efficacy of these FLT3 inhibitors and correlate it with patient outcomes.
Results
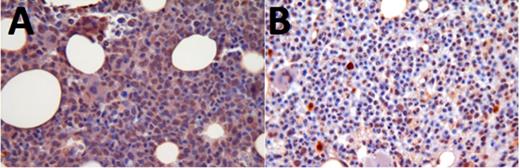
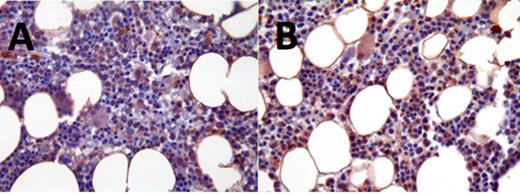
The different FLT3 compounds tested had a heterogenous effect on activated FLT3 levels. In some patients, ASP2215 caused a decrease in levels of activated FLT3 (indicated by brown nuclear staining), which corresponded to a decrease in leukemic blasts (Fig 1). Other patients, however, showed similar amounts of activated FLT3 both before and after treatment, which corresponded with no significant change in leukemic blasts. (Fig 2).
Other FLT3 inhibitors also showed differences in their effects. Midostaurin reduced activated FLT3 levels, which correlated with a positive clinical response. In contrast, one patient receiving quizartinibshowed little to no decrease in activated FLT3 levels, despite remaining in clinical remission. This suggests that this FLT3 inhibitor may have alternative targets, such as the tyrosine kinases AXL or LTK.
Conclusions
The heterogeneity in the responses to ASP2215, midostaurin, and quizartinib suggests that there may be other targets for these compounds that are not currently accounted for in the clinical studies. Immunohistochemicalmeasurements of activated FLT3 in bone marrow sections before and after treatment with FLT3 inhibitors was not predictive for clinical response.
Activated FLT3 levels in patient responsive to ASP2215; pre-treatment (A) and post-treatment (B)
Activated FLT3 levels in patient responsive to ASP2215; pre-treatment (A) and post-treatment (B)
Activated FLT3 levels in patient not responsive to ASP2215; pre-treatment (A) and post-treatment (B)
Activated FLT3 levels in patient not responsive to ASP2215; pre-treatment (A) and post-treatment (B)
Larson:Pfizer: Consultancy; Ariad: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal