Abstract
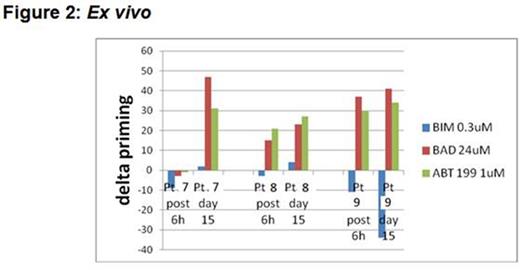
Recent studies have demonstrated promising preclinical efficacy for the combination of ibrutinib with venetoclax (formerly ABT-199/GDC-0199) in CLL; however, the biology underlying how ibrutinib enhances the sensitivity of CLL cells to BCL-2 antagonism by venetoclax has heretofore remained obscure. Our group previously developed BH3 profiling, a functional assay that determines the proximity of a cell to the threshold of apoptosis, a property that we call "mitochondrial priming". A new variant of this technique known as dynamic BH3 profiling (DBP) measures early changes in net pro-apoptotic signaling at the mitochondrion that are induced in cancer cells treated with drugs. This technique can be used to determine "delta priming", which reflects the degree of short term change in mitochondrial priming in response to various drug treatments. We hypothesized that DBP would allow us to determine how ibrutinib and venetoclax influence mitochondrial priming and anti-apoptotic dependence in primary CLL cells. Mononuclear cells were isolated from CLL patients and treated in vitro with ibrutinib and/or venetoclax. Co-culture was performed with the StromaNKtert cell line seeded at a 1:10 ratio relative to CLL cells. BH3 profiling was performed as previously described by measuring the release of cytochrome C on a BD FACS Fortessa. Cell viability was determined by AnnexinV/PI by FACS. Western blot analyses were performed by standard protocols with signals captured by an LAS 4000 imager. The student's paired t-test was used to compare drug treatments with a one-tailed p ≤ 0.05 considered significant. We performed DBP on samples from 6 previously untreated CLL patients and found that BIM BH3 peptide (a measure of general priming) induced delta priming of 10% or less in all 6 samples treated in vitro with ibrutinib, whereas all 6 samples treated with venetoclax had delta priming of 20% or greater (Figure 1A). The results observed with BAD BH3 peptide (a measure of BCL-2 dependence) were exactly the opposite (Figure 1B), suggesting that while venetoclax increases the overall level of mitochondrial priming of CLL cells, ibrutinib selectively increases the dependence of CLL cells on BCL-2. Consistent with this finding, samples treated in vitro with ibrutinib also had increased BAD protein expression by Western blot compared to samples treated with venetoclax. Importantly, we confirmed these findings ex vivo in primary CLL cells isolated from 3 patients treated with ibrutinib on the recently reported phase 3 PCYC-1112 (RESONATE) trial. In all 3 patients, delta priming with BAD BH3 peptide was increased by day 15 of treatment (Figure 2), with 2 patients showing increases as early as 6 hours post first dose. By Western blot, increased BAD protein was observed after ibrutinib treatment in 2 of these 3 patients. Cells from patients on ibrutinib were also found to be more sensitive to venetoclax by Annexin/PI compared to their pre-treatment samples. These data suggest that ibrutinib makes mitochondria more BCL-2 dependent, providing further justification for moving forward with clinical trials of the ibrutinib plus venetoclax combination in CLL.
Letai:AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Tetralogic: Consultancy, Research Funding. Davids:Janssen: Consultancy; Pharmacyclics: Consultancy; Genentech: Other: ad board.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal