Abstract

Introduction: This phase 1, open-label, randomized, crossover study was performed to test the bioequivalence and safety of an investigational, ready-to-dilute, rapid infusion (low volume) solution of bendamustine hydrochloride (test product [T]), and the approved bendamustine lyophilized powder formulation (reference product [R]). [ClinicalTrials.gov Identifier: NCT02162888]
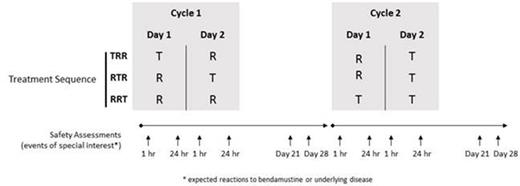
Methods: Patients were eligible to participate if they had a histologically confirmed diagnosis of any malignant disease (excluding chronic lymphocytic leukemia) for which no curative or standard therapy was available. All patients received bendamustine 120 mg/m2 intravenously as T (in 50 mL; 0.9% NaCl) over 10 min and R (in 500 mL; 0.9% NaCl) over 60 min on days 1 and 2 of two consecutive 28-day cycles. Patients were randomly assigned to one of three treatment sequences for the first three doses of study drug: TRR, RTR, RRT; T was given to all patients at cycle 2, day 2 (dose 4) (Figure). Safety was assessed throughout the 56-day treatment period, with events of special interest (expected reactions to bendamustine or underlying disease) recorded during and up to 1 h after administration; up to 24 h after administration; and on cycle 1 days 21 and 28; cycle 2 day 28; and at the end-of-study visit. Safety assessments included reported adverse events (AEs), Eastern Cooperative Oncology Group performance status, vital sign measurements, physical examination, and clinical laboratory assessments.
Results: A total of 83 patients were randomized to the 3 treatment sequences; 81 received at least one dose of study drug and comprised the safety population. Fifty-nine (71.1%) patients received four doses of study treatment, with a similar number (67%-77%) of patients in each treatment sequence arm. Reasons for discontinuation were AEs (4 patients), death (3 patients), insufficient therapeutic response (4 patients), lost to follow-up (1 patient), sponsor request (2 patients), investigator request (3 patients), withdrew consent (5 patients), and other (2 patients). The frequency of treatment-emergent AEs (TEAEs) by most recent study treatment are shown in the Table. The most commonly occurring AEs (> 10%) overall were nausea (R 25%, T 19%), fatigue (R 21%, T 22%), dehydration (R 12%, T 15%), decreased appetite (R 11%, T 15%), anemia (R 12%, T 15%), pyrexia (R 6%, T 16%), constipation (R 11%, T 11%), vomiting (R 11%, T 8%), dyspnea (R 5%, T 12%), hypokalemia (R 4%, T 12%), diarrhea (R 6%, T 10%), abdominal pain (R 5%, T 10%), and thrombocytopenia (R 2%, T 11%). The most common AEs occurring during the infusion and within 1 h after infusion were nausea (R 6%, T 8%) and fatigue (R 5%, T 6%); nausea (R 11%, T 9%) and fatigue (R 8%, T 9%) were also most common in the first 24 h. Infusion reactions were experienced by ≤ 2% of patients treated with either T or R. Serious AEs occurring in > 1 patient were abdominal pain (R 1%, T 5%), chest pain (R 2%, T 0), deep vein thrombosis (R 2%, T 0), dehydration (R 1%, T 1%), pericardial effusion (R 1%, T 1%), and vomiting (R 1%, T 3%). Six deaths were reported; the primary cause of death for all six patients was progressive disease.
Conclusions: No new safety signals were observed. The fact that all patients who received 4 doses received T as their final dose introduces a source of bias and may explain the increased overall number of TEAEs attributed to T.
Sponsor: Eagle Pharmaceuticals, Inc.
TEAEs by Most Recent Treatment (Safety Population)
| . | R n = 81 . | T n = 73 . | ||
|---|---|---|---|---|
| . | Events . | Patients n (%) . | Events . | Patients n (%) . |
| During or within 1 h after infusion | ||||
| All TEAEs | 40 | 26 (32.1) | 38 | 21 (28.8) |
| Grade 3-4 | 0 | 0 | 1 | 1 (1.4) |
| Serious AEs | 0 | 0 | 1 | 1 (1.4) |
| Within 24 h of infusion | ||||
| All TEAEs | 65 | 34 (42.0) | 50 | 23 (31.5) |
| Grade 3-4 | 5 | 4 (4.9) | 10 | 6 (8.2) |
| Serious AEs | 2 | 2 (2.5) | 5 | 2 (2.7) |
| Overall | ||||
| All TEAEs | 263 | 60 (74.1) | 348 | 49 (67.1) |
| Grade 3-4 | 46 | 19 (23.5) | 66 | 20 (27.4) |
| Serious AEs | 23 | 12 (14.8) | 12 | 12 (16.4) |
| . | R n = 81 . | T n = 73 . | ||
|---|---|---|---|---|
| . | Events . | Patients n (%) . | Events . | Patients n (%) . |
| During or within 1 h after infusion | ||||
| All TEAEs | 40 | 26 (32.1) | 38 | 21 (28.8) |
| Grade 3-4 | 0 | 0 | 1 | 1 (1.4) |
| Serious AEs | 0 | 0 | 1 | 1 (1.4) |
| Within 24 h of infusion | ||||
| All TEAEs | 65 | 34 (42.0) | 50 | 23 (31.5) |
| Grade 3-4 | 5 | 4 (4.9) | 10 | 6 (8.2) |
| Serious AEs | 2 | 2 (2.5) | 5 | 2 (2.7) |
| Overall | ||||
| All TEAEs | 263 | 60 (74.1) | 348 | 49 (67.1) |
| Grade 3-4 | 46 | 19 (23.5) | 66 | 20 (27.4) |
| Serious AEs | 23 | 12 (14.8) | 12 | 12 (16.4) |
Anthony:Eagle Pharmaceuticals, Inc.: Research Funding. Edenfield:Novartis, Astellas/Medivation: Speakers Bureau. Smith:Eagle Pharmaceuticals, Inc.: Employment. Hepner:Eagle Pharmaceuticals, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal