Abstract
Introduction: This phase 1, open-label, randomized, crossover study assessed the bioequivalence (BE) and safety of an investigational, ready-to-dilute, rapid infusion, low-volume solution of bendamustine hydrochloride (test product [T]) and the approved bendamustine lyophilized powder formulation (reference product [R]). [CT.gov ID NCT02162888]
Methods: Eligible patients (Pts) were aged 18 years or older with relapsed or refractory solid tumors or hematologic malignancies excluding chronic lymphocytic leukemia. All Pts received bendamustine 120 mg/m2 intravenously as T (in 50 mL; 0.9% NaCl) over 10 min, and as R (in 500 mL; 0.9% NaCl) over 60 min on days 1 and 2 of two consecutive 28-day cycles. Patients were randomly assigned to 1 of 3 treatment sequences defining the first 3 doses of study drug: TRR, RTR, RRT. T was given to all Pts at cycle 2, day 2. For the first 3 doses, blood samples were collected prior to infusion; mid-infusion (T 5 min, R 30 min); at 5, 15, 30, and 45 min, and at 1, 1.5, 2, 3, 4, 5, and 8 h postinfusion; and 24 h from the start of infusion on day 1 of both cycles. The pharmacokinetic (PK) endpoints for BE were area under plasma concentration-vs-time curve (AUC) from time 0 to the last quantifiable sample collected (AUC0-t) and from time 0 to infinity (AUC0-oo), which were evaluated using a scaled average BE (SABE) method, appropriate for high variability drugs. Other PK endpoints were maximum plasma concentration (Cmax), time to Cmax (tmax), and elimination half-life (t1/2). Safety was assessed by reported adverse events (AEs), Eastern Cooperative Oncology Group performance status, physical examination, and laboratory values throughout the 56-day study period.
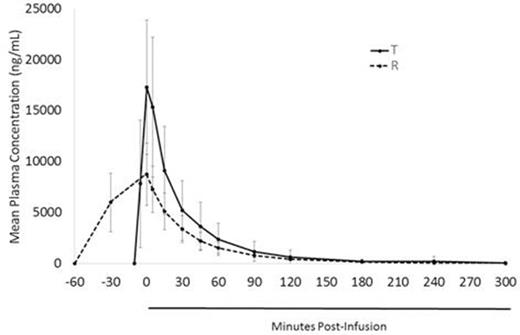
Results: A total of 83 Pts were randomized to the 3 treatment sequences; 81 received at least one dose of study drug and comprised the safety population; 60 received 3 doses (required for BE analysis). BE analyses were conducted for AUC in this population, and for other patient subsets based on dosing and sample collections deemed evaluable and complete. Here, PK results are presented for the 38 Pts meeting all BE inclusion criteria (Table 1). The BE of T and R was assessed by Reference-SABE for AUC as intra-subject variability for R (SWR) was >0.3 (Table 2). Mean concentration-vs-time is presented in the Figure. The AUC and t1/2 were similar in Pts treated with T and R. Cmax was higher and tmax was shorter in Pts treated with T, consistent with the faster infusion of bendamustine. The overall safety profiles of T and R were similar (Table 3), with serious AEs (SAEs) in 28% of Pts and 6 deaths (all attributable to disease progression). AEs occurring within 24 h of treatment were similar in type and frequency; the only AEs occurring in ≥3% of Pts with either treatment during this period were nausea (R 11%, T 9%), fatigue (R 8%, T 9%), vomiting (R 3%, T 4%), and constipation (R 4%, T 3%).
Conclusions: BE of the two bendamustine formulations was demonstrated for AUCs as the upper critical values were <0 and the point estimates of T/R geometric mean ratio fell within 0.80 to 1.25 inclusive. Differences in Cmax and tmax were anticipated from the different infusion rates for the T and R. The safety profile of the two drugs was comparable with no new safety signals. Reported AEs were either known effects of bendamustine or presumed to be related to underlying disease.
Sponsor: Eagle Pharmaceuticals, Inc.
Summary of PK Parameters
| Parameter . | T n = 38 . | R n = 38 . |
|---|---|---|
| AUC0-t, ng·h/mL (% CV) | 10339.21 (49.3) | 10514.87 (55.9) |
| AUC0-¥, ng·h/mL (% CV) | 10369.74 (49.2) | 10527.76 (55.8) |
| tmax, h (range) | 0.18 (0.1-0.4) | 1.0 (0.5-1.3) |
| Cmax, ng/mL (% CV) | 19158.16 (33.5) | 8868.42 (47.4) |
| t1/2, h (% CV) | 0.65 (37.3) | 0.60 (30.3) |
| Parameter . | T n = 38 . | R n = 38 . |
|---|---|---|
| AUC0-t, ng·h/mL (% CV) | 10339.21 (49.3) | 10514.87 (55.9) |
| AUC0-¥, ng·h/mL (% CV) | 10369.74 (49.2) | 10527.76 (55.8) |
| tmax, h (range) | 0.18 (0.1-0.4) | 1.0 (0.5-1.3) |
| Cmax, ng/mL (% CV) | 19158.16 (33.5) | 8868.42 (47.4) |
| t1/2, h (% CV) | 0.65 (37.3) | 0.60 (30.3) |
CV: coefficient of variation
Bioequivalence Analyses Results (n = 38)
| AUC (ng·h/mL) . | GM1 Test (T) . | GM1 Reference (R) . | T/R . | 90% Confidence Intervals . | Swr . | Upper Critical Value . |
|---|---|---|---|---|---|---|
| AUC0-t | 9143.72 | 9047.81 | 1.01 | 0.898-1.145 | 0.403 | -0.09 |
| AUC0--¥ | 9173.01 | 9062.77 | 1.02 | 0.899-1.146 | 0.402 | -0.09 |
| AUC (ng·h/mL) . | GM1 Test (T) . | GM1 Reference (R) . | T/R . | 90% Confidence Intervals . | Swr . | Upper Critical Value . |
|---|---|---|---|---|---|---|
| AUC0-t | 9143.72 | 9047.81 | 1.01 | 0.898-1.145 | 0.403 | -0.09 |
| AUC0--¥ | 9173.01 | 9062.77 | 1.02 | 0.899-1.146 | 0.402 | -0.09 |
GM: geometric mean, Swr: within-patient standard deviation of the reference product1 by SABE
Summary of AEs
| Patients n (%) . | T n = 73 . | R n = 81 . | Total n = 81 . |
|---|---|---|---|
| Overall | |||
| AEs | 49 (67) | 60 (74) | 76 (94) |
| SAEs | 12 (16) | 12 (15) | 23 (28) |
| Deaths* | 5 (7) | 1 (1) | 6 (7) |
| Occurring within 24 h of infusion | |||
| AEs | 23 (32) | 34 (42) | 49 (61) |
| SAEs | 2 (3) | 2 (3) | 4 (5) |
| Deaths | 0 | 0 | 0 |
| Patients n (%) . | T n = 73 . | R n = 81 . | Total n = 81 . |
|---|---|---|---|
| Overall | |||
| AEs | 49 (67) | 60 (74) | 76 (94) |
| SAEs | 12 (16) | 12 (15) | 23 (28) |
| Deaths* | 5 (7) | 1 (1) | 6 (7) |
| Occurring within 24 h of infusion | |||
| AEs | 23 (32) | 34 (42) | 49 (61) |
| SAEs | 2 (3) | 2 (3) | 4 (5) |
| Deaths | 0 | 0 | 0 |
*All attributable to disease progression
Mean (± SD) bendamustine plasma concentration versus time for rapid-infusion test (T) and reference (R) formulations
Mean (± SD) bendamustine plasma concentration versus time for rapid-infusion test (T) and reference (R) formulations
Edenfield:Novartis, Astellas/Medivation: Speakers Bureau. Anthony:Eagle Pharmaceuticals, Inc.: Research Funding. Mutch:Eagle Pharmaceuticals, Inc.: Employment. Chanas:Eagle Pharmaceuticals, Inc.: Employment. Smith:Eagle Pharmaceuticals, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal