Abstract
Background: The incidence of CML increases with age and also the disease characteristics vary with age. However, whether age has any impact on molecular response (MR) with TKIs or not has not been clearly established.
Objective: To assess the effect of age on MR and adverse events (AEs) with nilotinib (NIL) in patients (pts) with CML in the ENEST1st (NCT01061177) phase 3b trial.
Patients and Methods: ENEST1st is an open-label study of NIL 300 mg BID in adults with newly diagnosed CML-CP. Treated pts with typical transcripts and ≤ 3 months of prior imatinib (IM) therapy were included in the sub-analysis and were stratified according to age (> 65 years and ≤ 65 years). Primary endpoint was rate of MR4 (defined as BCR-ABL ≤ 0.01% on the International Scale [BCR-ABLIS ] or undetectable BCR-ABL in cDNA with ≥ 10,000 ABL transcripts) at 18 months.
Results: Of the 1089 pts (median age, 53 years; range, 18 - 91 years) enrolled into the study, 1052 had b2a2 and/or b3a2 BCR-ABL transcripts and were treated with IM for ≤ 3 months. Sokal risk scores were low, intermediate, and high in 34.9%, 37.5%, and 18.1% of pts, respectively (9.5% missing).
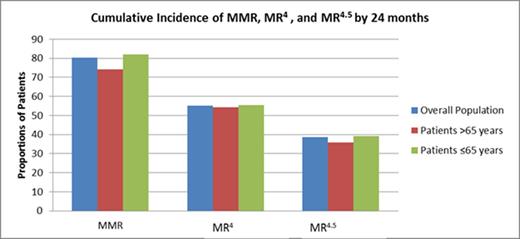
The rate of MR4 at 18 months in all treated pts with typical transcripts and ≤ 3 months of prior IM (n = 1052) was 38.4% (95% CI, 35.5% - 41.3%). The cumulative rates of MR at 24 months are shown in the figure.
At 12 months the rates of MR4 and MR4.5 were 30.8% and 15.3% respectively. Among pts who were in MR4 and MR4.5 at 12 months, 60.8% and 33.9% pts were able to maintain continuous MR4 and MR4.5 until Month 24, with no documented loss of MR4 and MR4.5 between 12 and 24 months, respectively.
Among all pts treated (n=1089), six pts (0.6%) progressed to accelerated phase/blast crisis (AP/BC) on study; 13 pts (1.2%) died by 24 months, including one due to CML progression (16 month after study drug discontinuation). The most common AEs of any cause were rash (21.4%), pruritus (16.5%), and headache (15.2%).
Subgroup-analysis by age: As per the WHO guidelines, pts > 65 years are defined as older. Accordingly, for this sub-analysis, pts with typical transcripts and treated with IM for ≤ 3 months (n = 1052) were stratified into two subgroups: 851 pts (80.9%) were ≤ 65 years of age and 201 pts (19.1%) were > 65 years old at the time of enrollment. The median age at enrollment was 71 (range 66 - 91) and 48 (range 18 - 65) years in the old and young group, respectively. Among old pts, Sokal risk scores were low, intermediate, and high in 4.5%, 61.2%, and 23.4% of pts, respectively (10.9% missing). Among young pts, Sokal risk scores were low, intermediate, and high in 42.1%, 32.0%, and 16.9% of pts, respectively (9.0% missing).
Cumulative incidence of MR4 by 18 months was 48.8% (95% CI, 45.4% - 52.1%) among younger pts and 48.3% (95% CI, 41.4% - 55.2%) in older pts. Rate of MR4 by 18 months among younger pts with low, intermediate, and high Sokal score was 53.6%, 45.2%, and 35.4%, respectively. Rate of MR4 by 18 months among older pts with low, intermediate, and high Sokal score was 44.4%, 49.6%, and 44.7%, respectively.
The incidence rate of MR4.5 by 18 months in younger pts and older pts was 32.5% and 28.4%, respectively.
Most common AEs experienced by younger patients were rash (23%), headache (17%), pruritus (16%), and fatigue (14%). In older population, the list of most common AEs includes pruritus (19%), nausea (18%), and rash (14%) (Table).
Conclusions: In this study, frontline NIL yielded equivalent rates of molecular responses in older and younger pts with a similar safety profile.
| Adverse Event . | Patients ≤65 years (n = 851) % . | Patients >65 years (n = 201) % . |
|---|---|---|
| Rash | 23.2 | 13.8 |
| Headache | 17.1 | 7.6 |
| Pruritus | 16.0 | 18.6 |
| Fatigue | 14.0 | 13.3 |
| Thrombocytopenia | 11.0 | 7.6 |
| Nasopharyngitis | 10.8 | 8.6 |
| Alopecia | 10.8 | 9.5 |
| Nausea | 9.8 | 17.6 |
| Diarrhea | 8.0 | 11.4 |
| Asthenia | 8.4 | 11.0 |
| Anemia | 5.1 | 10.5 |
| Abdominal pain | 6.9 | 10.5 |
| Dry skin | 8.2 | 10.0 |
| Adverse Event . | Patients ≤65 years (n = 851) % . | Patients >65 years (n = 201) % . |
|---|---|---|
| Rash | 23.2 | 13.8 |
| Headache | 17.1 | 7.6 |
| Pruritus | 16.0 | 18.6 |
| Fatigue | 14.0 | 13.3 |
| Thrombocytopenia | 11.0 | 7.6 |
| Nasopharyngitis | 10.8 | 8.6 |
| Alopecia | 10.8 | 9.5 |
| Nausea | 9.8 | 17.6 |
| Diarrhea | 8.0 | 11.4 |
| Asthenia | 8.4 | 11.0 |
| Anemia | 5.1 | 10.5 |
| Abdominal pain | 6.9 | 10.5 |
| Dry skin | 8.2 | 10.0 |
Giles:Novartis: Consultancy, Honoraria, Research Funding. Rea:Novartis: Honoraria; BMS: Honoraria; Pfizer: Honoraria; Ariad: Honoraria. Baccarani:NOVARTIS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ARIAD Pharmaceuticals, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Cross:Ariad: Consultancy, Honoraria, Research Funding; Qiagen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Steegmann:Bristol-Myers Squibb: Honoraria, Research Funding; Ariad: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding. Griskevicius:Novartis: Honoraria, Research Funding. Coriu:PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ossenkoppele:Pfizer: Honoraria, Research Funding; ARIAD: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Mahon:Pfizer: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Müller:Ariad: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding. Hellmann:BMS: Research Funding; Novartis: Research Funding. Porkka:Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Novartis: Honoraria; Pfizer: Honoraria. Brümmendorf:Novartis: Consultancy, Patents & Royalties, Research Funding. Gastl:AOP Orphan: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Tubazio:Novartis: Employment. Pellegrino:Novartis: Employment. Dezzani:Novartis: Employment. Rosti:Bristol Myers Squibb: Honoraria, Research Funding, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau. Hochhaus:Novartis: Honoraria, Research Funding; ARIAD: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal