Abstract
Background: Bone marrow stroma provides a favorable microenvironmental niche for ALL cell survival. We and others have demonstrated that bone marrow stromal cells contribute to prevention of apoptosis in ALL cells.
Objective: Identify potentially "drug-able" molecules derived from marrow stromal cells that contribute to prevention of ALL cell apoptosis.
Methods: We have developed an in vitro system to identify stromal gene products that deliver antiapoptotic signals to ALL cells. Primary human ALL cells are co-cultured with human bone marrow stromal cells. We manipulate stromal cells with siRNA directed against candidate stromal cell genes. Two days later the siRNA is washed out of culture and primary ALL cells are added to the stromal cells. Controls include irrelevant siRNA. Five days later we measure viability and apoptosis in ALL cells by flow cytometry.
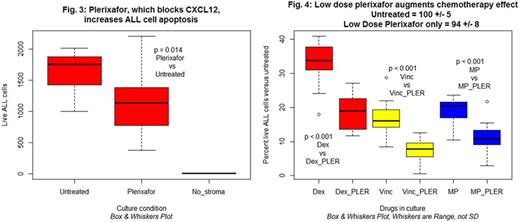
Results: (1) Knockdown of stroma cell CXCL12 or TGFBI reduces ALL survival. We performed global gene expression analysis upon human marrow stromal cells using RNASeq technology. Using bioinformatic approaches we are selecting some of the expressed stromal genes as candidates for the molecular mechanisms by which stromal cells prevent ALL apoptosis. We present preliminary results for two of our early candidates. (A) CXCL12 is a paracrine chemokine known to have activity in the marrow microenvironment upon hematopoietic cells and we hypothesized it may participate in the effect. Knockdown of CXCL12 with siRNA increased ALL cell death in the co-culture system. As measured by quantitative reverse transcriptase PCR stromal cell CXCL12 mRNA was reduced approximately 75% by siRNA treatment. Figure 1 displays representative results of the impact of CXCL12 knockdown in stromal cell on the survival of ALL cells in the coculture. The magnitude of effect was ~40% increase in ALL cell death. (B) TGFBI (transforming growth factor beta induced) is expressed by stromal cells. The gene is involved in cell-collagen interactions and we hypothesized it played a role. siRNA reduced stromal gene expression by about 90%. Figure 2 displays representative results in which ALL cell death increased by about 50%. (2) Validation of results using inhibitors to CXCL12. The gene knockdown experiments suggested a potential role for CXCL12 in prevention of ALL cell apoptosis. To further test this we tested the effect of plerixafor, a specific inhibitor of CXCL12/CXCR4 interactions, on survival of ALL. ALL cells express CXCR4. In a dose dependent manner (25 - 400 micromolar) we observed a 31-39% reduction in ALL survival in stromal co-cultures including plerixafor. Figure 3 depicts representative results with plerixafor 200 micromolar. We are evaluating small molecules to block TGFBI. (3) Potential augmentation of chemotherapy drug effects on ALL. We hypothesize that interference with stromal cell molecules that prevent apoptosis in ALL cells may increase the effectiveness of conventional antileukemia drugs. In our stromal cell/ALL coculture system we have identified the effective in vitro concentrations of the most commonly used ALL drugs. We measured the impact of combination of low dose plerixafor (LD10) and these individual drugs (used at approximately the LD50 concentrations). Figure 4 demonstrates increased antileukemia effects related to plerixafor for dexamethasone, vincristine, and 6-mercaptopurine. Results are plotted as a percentage of ALL cells surviving in the absence of any drugs. The low dose plerixafor alone control did not produce a statistically significant reduction in ALL survival.
Conclusions: Marrow stromal cell-produced CXCL12 may contribute to prevention of apoptosis in human ALL cells. Pharmacological interference with its effect may enhance the effectiveness of some conventional chemotherapy drugs. Marrow stromal cell-produced TGFBI may also contribute to prevention of apoptosis in human ALL cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal