Abstract
Introduction
In robust hospital transfusion services, transfusion reaction reporting triggers a structured response to the assessment, diagnosis, and clinical management of the individual. At a population level, the feedback loop of hemovigilance permits the perception of signals applicable to donors, material production, patterns of use, infusion care, and recipient vulnerabilities. Transfusion reaction reporting therefore aims to improve the quality of patient care and safety in transfusion, which remains one of the most commonly performed procedures in medicine today. Large variations between passive/retrospective or active/prospective systems imply underreporting. Reasons for this may lie in unawareness of, nihilism on, or obstacles to this duty. In 2009, our center transitioned from a paper-based to an electronic reporting system (ERS) for suspected patient reaction events (PREs). This study sought to determine the impact of this change on PRE reporting rates.
Methods
This study was conducted in Toronto, Canada at the University Health Network, a 4-site, 767-bed, ternary care hospital with high transfusion activity (2014: 60,000 component and 30,000 derivative dispensations). In 05/2009, hardcopy mountsheets for transfusion labels were revised to provide space for recording corresponding vital signs, with instructions on PRE reporting. Medical director PRE review followed with event documentation in a transfusion laboratory database (recording imputability, reaction type, severity, and implicated product(s)). An Acute Transfusion Reaction policy was also developed to protocolize and further streamline the approach to various reactions, but was not implemented across all sites until 11/2009. At this time, electronic PRE reporting went live in the existing electronic medical record, with medical director review hereafter culminating in uploaded case conclusions. Technical tutorials on healthcare worker reporting spanned several months before implementation, without emphasizing the theory or evidence-based value of hemovigilance. The quantity and characteristics of reactions pre-/post-ERS implementation were compared.
Results
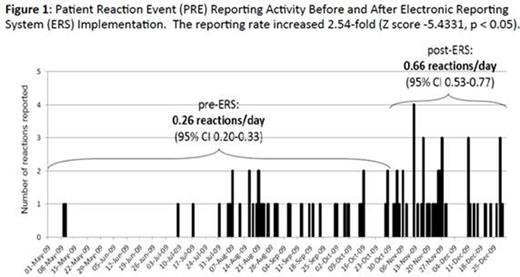
Prior to the ERS option (5/2009-11/2009), the reported PRE rate was 0.26/day. Subsequent to launch (11/2009-12/2009), the reported PRE rate was 0.66/day, representing a 2.54 fold increase (p<0.05) (Figure 1). This nearly-trebled rate has been sustained throughout subsequent years: 01/2010-12/2010: 0.88/day; 01/2011-12/2011: 0.87/day; 01/2012-12/2012: 0.87/day; 01/2013-12/2013: 0.88/day; 01/2014-12/2014: 0.93/day. The distribution of PRE conclusions pre-ERS was: febrile non-hemolytic transfusion reaction (FNHTR) 40%; unrelated to transfusion (UTR) 34%; allergic transfusion reaction (ATR) 7.3%; query bacterial contamination (BaCON) 4.9%; transfusion related acute lung injury (TRALI) 3.6% and transfusion related circulatory overload (TACO) 2.4%. The distribution of PRE conclusions after ERS (11/2009-12/2014) was: ATR 28.8%; UTR 28.7%; FNHTR 19.9%; TACO 8.6%; transfusion associated dyspnea 4.7%; pain 3.2%; query BaCON 2.3% and TRALI 1.7%.
Conclusions
Our data demonstrate that PRE reporting significantly increased and was sustained after the implementation of an ERS. This finding suggests that despite a dearth of strategies to address underreporting, the solution may lie in removing disincentives while facilitating action in familiar practice platforms. Two other studies investigated the implementation of an ERS for transfusion reaction reporting (Fujihara H. et al. 2015; Yeh, S et al. 2011), with one confounded by a significant increase in transfusion rates in the post-ERS period. In contrast, our denominator of blood utilization has been stable or decreasing across sites over the last five years, with 3% of product recipients nevertheless experiencing a PRE. Despite the significant increase in reported PREs, we did not see an increase in UTRs (34% vs 25%) to account for the difference, arguing against "junk inflations," while rather suggesting that reporter suspicions generally concur with specialist conclusions on transfusion imputability. Given the importance of accurate transfusion reaction reporting for patient safety, we suggest that this strategy be considered by other centers to improve reporting activity with its potential downstream benefits.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal