Abstract
BACKGROUND: Acquired hemophilia A (AHA) results from auto-antibodies that neutralize factor VIII (FVIII) coagulant function. AHA is rare, usually occurring late in life, and occasionally post-partum. It is most often idiopathic, but can be associated with malignancy, autoimmunity, or drugs. In contrast to congenital hemophilia, AHA generally manifests as mucocutaneous bleeding, with poor correlation to antibody titer or residual FVIII activity. Management goals are the achievement of hemostasis and antibody eradication. Here we describe an unusual case of AHA and concomitant factor XIII consumption in a young nulliparous woman.
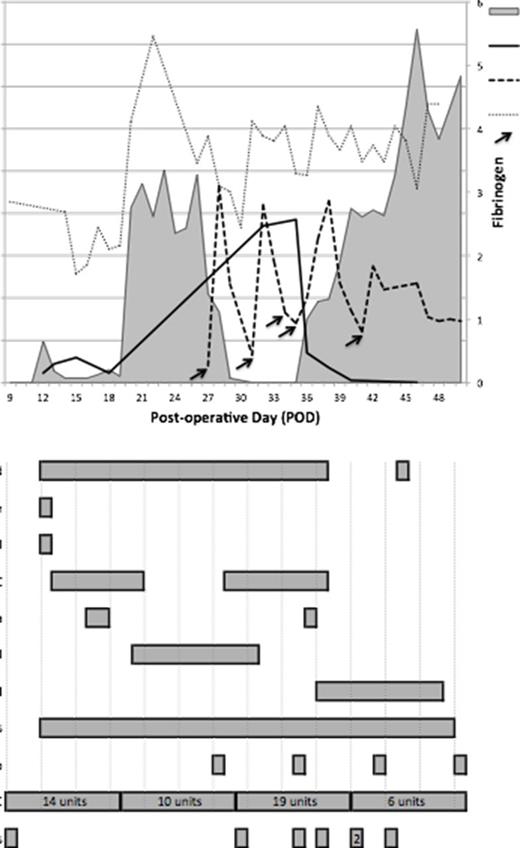
CASE REPORT: A 24-year-old woman with a symptomatic congenital choledochal cyst underwent a Roux-en-Y bypass, bile duct resection and cholecystectomy. Abdominal sepsis ensued from extended spectrum beta-lactamase Klebsiella, with ertapenem initiation on post-operative day (POD) 5. On POD6 she developed severe intra-abdominal bleeding associated with prolongation of the activated partial thromboplastin time (aPTT) from 35 seconds (pre-operative) to 40 seconds (reference interval 28-35 seconds). On POD10, FVIII activity and FVIII inhibitor titer, using the modified Nijmegen Bethesda assay, were measured at 0.08 U/ml and 4.5 BU/ml, respectively. In addition to supportive transfusion therapy, the patient received a sequence of desmopressin (0.3 mcg/kg IV), recombinant FVIII (50 U/kg), and activated prothrombin complex concentrate (aPCC) (50-75 U/kg IV q8h) with tranexamic acid (10-20 mg/kg IV q8h) from POD13-20. Prednisone was concomitantly initiated (1.5 mg/kg/day) (Figure 1). Bleeding persisted despite reduction in clotting time on rotational thromboelastometry post-aPCC. As neither intensified aPCC nor recombinant factor VIIa (rFVIIa) (80 mcg/kg IV q2h) provided substantial benefit, recombinant porcine factor VIII (rpFVIII) was administered from POD20-28 (100 U/kg IV qd). RpFVIII resulted in hemostatic improvement until anti-porcine FVIII antibodies developed on POD27. On POD28, she was transferred to the local hemophilia treatment center where complete factor analysis identified concurrent FXIII deficiency [0.08 U/ml] using a latex immunoassay. FXIII inhibitor analysis using a chromogenic assay was negative. The peak FVIII inhibitor titer and nadir FVIII activity were 74 BU/ml and <0.01 U/ml, respectively. APCC was resumed [POD28-37] and plasma derived factor XIII (pdFXIII) was given every 4 days to maintain FXIII >0.20 U/ml. The patient was also treated with rituximab [POD28,36,43,50]. On POD37, massive intra-abdominal bleeding prompted sequential aPCC, rFVIIa and pd von Willebrand factor (VWF):FVIII concentrate. Once the FVIII inhibitor titer began to wane, bypassing therapy was discontinued and she was managed solely with pdVWF:FVIII. On POD47 all FVIII-related support was discontinued, as the FVIII inhibitor was undetectable and endogenous FVIII normalized. Her FXIII consumption and requirement for FXIII replacement, however, persisted.
DISCUSSION: The unusual features of this case of AHA include the patient's young age, the possible triggers for the FVIII inhibitor (extensive abdominal surgery, sepsis, and/or carbapenem use), and concomitant FXIII deficiency. We hypothesize that FXIII deficiency was secondary to consumption from the massive abdominal wound given its persistence despite normalization of hepatic function. Repeated life-threatening bleeding required trials of multiple hemostatic agents. Persistent and prolonged bleeding despite ongoing FVIII replacement should trigger investigation for a concomitant hemostatic deficiency. AHA is a rare condition, and treatment requires intensive monitoring, clinical experience and specialized laboratory support. Current guidelines are largely derived from expert opinion, and contemporary case descriptions for rare disorders are essential to advance options in best care.
Case summary outlining clinical course, factor activity levels, and management strategies
Case summary outlining clinical course, factor activity levels, and management strategies
St. Louis:Baxter/Baxalta: Consultancy. Sholzberg:CSL Behring: Honoraria, Research Funding; Baxalta: Honoraria, Research Funding; Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal