Abstract
Background: Follicular lymphoma (FL) is the most common subtype of indolent NHL. Current chemoimmunotherapeutic regimens used for FL are not curative. Rituximab, as a single-agent and in combination with chemotherapy, is a commonly used approach for frontline therapy of FL. Ibrutinib, a first-in-class, once-daily, oral inhibitor of Bruton's tyrosine kinase, has shown activity in a phase 1, first-in-human, dose-escalation trial in patients with relapsed/refractory FL, with an overall response rate (ORR) of 38% (6/16) including 3 complete responses [CRs]) (Advani, JCO 2013). In a multicenter, open-label phase 2 trial (PCYC-1125-CA), we evaluated the efficacy and safety of ibrutinib in combination with rituximab in treatment-naïve patients with FL.
Methods: Patients with treatment-naïve, histologically confirmed FL (Grade 1, 2 and 3a, stage II-IV disease) received oral ibrutinib 560 mg once daily until progressive disease (PD) or unacceptable toxicity, combined with rituximab 375 mg/m2 IV once weekly for 4 doses for the first 4 weeks of the study. The primary endpoint was ORR (2007 IWG criteria) as assessed by investigators. Secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
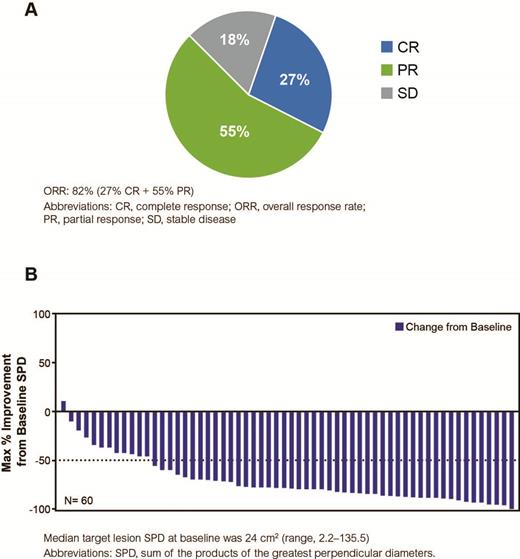
Results: Among 60 treated patients in study Arm 1, the median age was 58 years (range, 32-84), with 30% of patients aged ≥65 years, and 98% of patients with an Eastern Cooperative Oncology Group performance status of 0-1. At baseline, 80% of patients had Stage III/IV disease, and 10% of patients had grade 3a FL. The mean duration of treatment on ibrutinib was 9.2 months. At a median follow-up of 10.2 months (range, 1.2-16.2), the investigator-assessed ORR was 82% (95% CI: 70.1-89.4), with a CR rate of 27% and PR rate of 55% in all treated patients (Figure). The median time to best response was 2.7 months (range, 1.1-8.3). Median PFS, OS, and DOR are not reached as a result of a small number of PD (n=5) and death (n=1) events. Any-grade adverse events (AEs; ≥20%) included fatigue (63%), diarrhea (50%), nausea (42%), constipation (28%), headache (27%), maculopapular rash (27%), myalgia (23%), vomiting (23%), cough (22%), infusion-related reaction (22%), and dry eye (20%). Grade ≥3 AEs occurred in 43% of patients, and those occurring in >1 patient included fatigue and maculopapular rash (5% each), and neutropenia and hypertension (3% each). There was 1 death several months after study discontinuation due to Hodgkin's lymphoma. Serious AEs (SAEs) occurred in 13% of patients (12% grade 3 or 4). Any-grade bleeding was reported in 22% of patients, with only 1 grade 2 bleeding event (petechiae); all other events were grade 1. Atrial fibrillation ≥grade 3 occurred in 1 patient. Secondary malignancies were reported in 4 patients: Hodgkin's lymphoma (n=1; grade 3 and 5); fallopian tube cancer (n=1; grade 3); melanocytic nevus (n=1; grade 1); and basal cell carcinoma (n=1; grade 2). Overall, 28% of patients discontinued ibrutinib in the trial (AEs: 12%; PD: 8%; patient decision: 5%; and investigator decision: 3%). At the time of analysis, 72% of patients continued treatment. Overall, the study treatment was well tolerated.
Conclusions: In treatment-naïve patients with FL, ibrutinib combined with 4 cycles of rituximab demonstrates robust clinical activity with a high overall response rate. The combination is well tolerated; AEs were primarily grade 1-2 and as expected based on experience with single-agent ibrutinib and previously tested ibrutinib combinations.
Best Overall Response Rates in All Treated Patients (N=60) and Maximum Percentage Improvement from Baseline SPD (N=60)
Best Overall Response Rates in All Treated Patients (N=60) and Maximum Percentage Improvement from Baseline SPD (N=60)
Off Label Use: Ibrutinib for follicular lymphoma. Nastoupil:AbbVie: Research Funding; TG Therapeutics: Research Funding; Celgene: Honoraria; Genentech: Honoraria; Janssen: Research Funding. Knapp:Celgene: Research Funding; Heron Pharmaceuticals: Other: Travel expenses, Research Funding; Merck: Research Funding; Seattle Genetics, Inc.: Research Funding; Takeda Pharmaceuticals International Co.: Research Funding; Brystol-Myers Squibb: Research Funding; Genentech: Honoraria, Other: Travel expenses, Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; EMD Serono: Research Funding. Flinn:Cephalon, Inc; Teva Pharmaceutical Industries Ltd; Genentech, inc; Gilead: Research Funding. Chen:Genentech: Consultancy; Seattle Genetics: Consultancy, Research Funding; Janssen: Consultancy; Gilead: Consultancy. Bhatia:CHOP LLC: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria. Martin:Genentech, Inc.: Consultancy, Honoraria, Other: TRAVEL, ACCOMODATIONS, EXPENSES, Speakers Bureau; Janssen: Honoraria, Other: TRAVEL, ACCOMODATIONS, EXPENSES; Celgene: Consultancy, Honoraria, Other: TRAVEL, ACCOMODATIONS, EXPENSES; Gilead: Consultancy. Suzuki:Pharmacyclics LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Beaupre:Pharmacyclics LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Neuenburg:Pharmacyclics LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal