Abstract
Background: Atypical hemolytic uremic syndrome (aHUS) is a rare, genetic, life-threatening disease predominantly caused by chronic, uncontrolled complement activation that leads to thrombotic microangiopathy and renal and other end-organ damage. The aHUS Registry, established in April 2012, is an observational, noninterventional, multicenter, global initiative to collect information on patient outcomes regardless of treatment approach. It facilitates availability of follow-up data for eculizumab.
Methods: Patients with clinical diagnoses of aHUS (irrespective of identified complement abnormality or treatment) are eligible. Demographic, medical/disease history, and treatment outcomes data are collected at enrollment and prospectively thereafter.
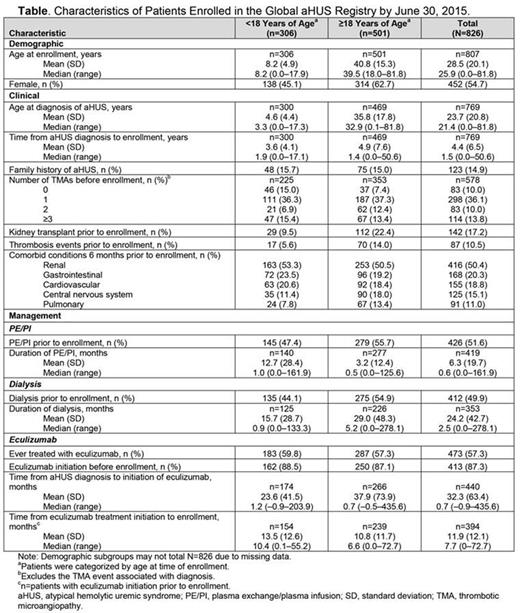
Results: By June 30, 2015, 826 patients enrolled (Table). Overall, 54.7% of patients, including 45.1% of pediatric and 62.7% of adult patients, were female. Patients were most commonly enrolled after their first TMA event. Thrombosis occurred more frequently in adult than pediatric patients. Nonrenal conditions, including gastrointestinal, cardiovascular, central nervous system, and pulmonary, were common in both age groups and occurred in 11.0%‒20.3% overall. Eculizumab was administered to 57.3% of patients, of whom 87.3% were treated prior to enrollment.
Conclusions: Registry baseline characteristics demonstrate differences between pediatric and adult patients with aHUS, notably frequencies of thrombosis. Nonrenal conditions are frequent in both age groups. Ongoing and future analyses will further enhance understanding of aHUS history and progression. Additional clinical sites are encouraged to enroll patients to facilitate knowledge acquisition and optimization of patient care and quality of life.
Licht:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Achillon: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Ardissino:Alexion Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ariceta:Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cohen:Astellas: Consultancy; Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Merck: Consultancy; Genentech: Research Funding. Gasteyger:Alexion Pharma International SàRL: Employment, Equity Ownership. Greenbaum:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ogawa:Alexion Pharmaceuticals: Employment, Equity Ownership. Kupelian:Alexion Pharmaceuticals: Employment, Equity Ownership. Schaefer:Alexion Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees. Vande Walle:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Frémeaux-Bacchi:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal