Abstract
Neutropenia is common in patients receiving myelotoxic chemotherapy. BenegrastimTM(F-627), a recombinant human G-CSF dimer, is an once-per-cycle therapy for prophylactic neutropenia in cancer patients after chemotherapy. Two phase I dose finding studies of benegrastim were conducted in Chinese women with stage I-IV breast cancer receiving myelotoxic chemotherapy. The aims of the studies were to evaluate the safety profile and pharmacokinetics (PK) and pharmacodynamics (PD) properties of benegrastim.
In the first study, a total of 18 patients were enrolled to receive 3 sequential dose levels of benegrastim at 80, 240 and 360 µg/kg (n=6). The patients received epirubicin/cyclophosphamide (EC) chemotherapy on day one. Benegrastim was administered to patients on day 3 by SC injection once per chemotherapy cycle for up to 4 cycles. In the second study, 15 patients were sequentially assigned to 240 µg/kg (n=7) and 320 µg/kg (n=8) of benegrastim. These patients received doxorubicin, docetaxel and cyclophosphamide (TAC) on day one, benegrastim was administered on day 2 by SC injection for up to 6 cycles. The PK/PD properties of benegrastim were evaluated in cycle one and cycle three.
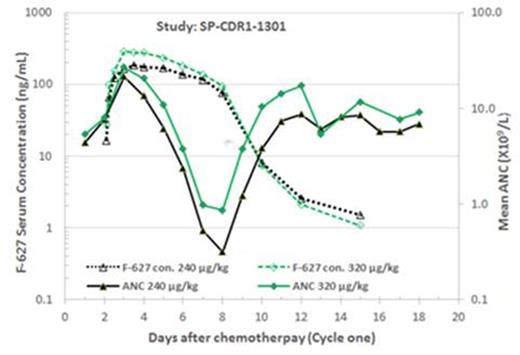
Benegrastim was well tolerated in both clinical studies. The treatment emergent adverse events related to benegrastim were back pain, arthralgia, musculoskeletal pain, and rash, commonly seen in rhG-CSF therapy. The PK/PD result in patients receiving TAC in the first chemotherapy cycle is shown in Fig 1. Dose-dependent benegrastim exposure and ANC increase were demonstrated in these two clinical trials. In breast cancer patients receiving EC or TAC, benegrastim showed a non-linear pharmacokinetics. Benegrastim shortened the duration of neutropenia post chemotherapy in a dose dependent manner. In conclusion, benegrastim may provide an alternative approach to manage neutropenia, especially, severe neutropenia in patients after chemotherapy.
PK/PD of F-627 (benegrastim) in Chinese breast cancer patients receiving TAC in the first chemotherapy cycle.
PK/PD of F-627 (benegrastim) in Chinese breast cancer patients receiving TAC in the first chemotherapy cycle.
Cao:Generon (Shanghai) Corporation Ltd.: Research Funding. Yuan:Generon (Shanghai) Corporation Ltd.: Research Funding. Huang:Generon (Shanghai) Corporation Ltd.: Employment. Ji:Generon (Shanghai) Corporation Ltd.: Research Funding. Peng:Generon (Shanghai) Corporation Ltd.: Research Funding. Yan:Generon (Shanghai) Corporation Ltd.: Employment. Tang:Generon (Shanghai) Corporation Ltd.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal