Abstract
Aplastic anemia (AA) is a common hematologic disease characterized by hematopoietic failure of the bone marrow and pancytopenia of the peripheral blood. Some patients with AA suffer from transfusion-induced iron overload secondary to long-term blood transfusions due to moderate and severe anemia. Topics related to iron overload and chelation therapy have recently become important in hematologic diseases, especially in hematopoietic failure. However, no animal model of AA complicated by iron overload has been developed, which affects drug research and development.
The purpose of our study is to establish a mouse model of aplastic anemia complicated by iron overload. We firstly controlled the total dose of iron supplements (2.0 *103 mg/kg) and compared how serum iron and ferritin levels varied among different dosage regimens to determine the best dosage and duration for the development of an iron overload model in mice (we finally chose the 200 mg/w/kg * 10 weeks). A composite model of AA was successfully established on the principle of immune-mediated bone marrow failure: DBA/2 mice were euthanized by cervical dislocation and the thymuses were taken out using aseptic techniques for the preparation of cell suspensions of 5 * 106/mL; Balb/c mouse were given whole-body irradiation with 60Co 6.0 Gy at 1 Gy/min, after which 0.2 mL of the prepared cell suspensions was injected into each mouse via the caudal vein within 4 h. We then compared the differences in liver volume, peripheral hemogram, bone marrow pathology, serum iron, serum ferritin, pathological iron deposition in multiple organs (liver, bone marrow, spleen), liver hepcidin, bone morphogenetic protein 6 (BMP6), SMAD family member 4 (SMAD4), transferrin receptor 2 (TfR2) protein, and mRNA expression levels among the Normal Control, AA, Iron Overload, and Composite Model groups to validate the composite model and to explore the pathogenesis and features of iron overload in the composite model.
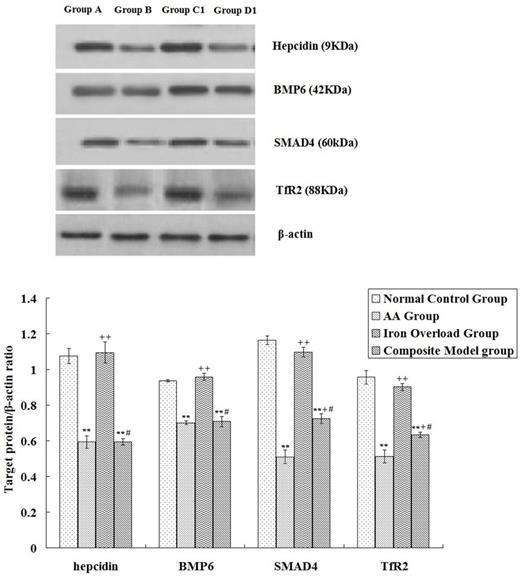
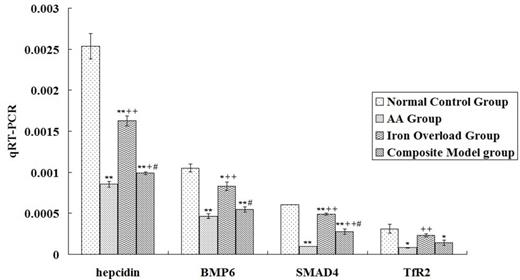
The results indicated significant abnormalities in iron metabolism parameters in mice with AA, which was reflected in the significant decrease of hepcidin expression in the liver (P < 0.01) that basically paralleled the changes in BMP6, SMAD4, and TfR2. Iron Overload Group had a suppressed hepcidin, BMP6, and SMAD4 mRNA expressions in liver, but these parameters were higher than in the AA group (P < 0.01). Association with iron overload would not further downregulate the negative parameters of iron deposition in mice with AA, and SMAD4 and TfR2 protein levels and hepcidin and SMAD4 mRNA expression levels were lower in the AA group than in the Composite Model group (P < 0.01 or P < 0.05). (see Fig.1 and Fig.2)
The established model is basically consistent with the clinical manifestations and pathogenesis of AA complicated by transfusion-induced iron overload. This successful establishment will help in the screening of iron chelation drugs and studies on pharmacological mechanisms.
Western-blot a nalysis of hepcidin, BMP6, SMAD4 and TfR2 expression among groups
Western-blot a nalysis of hepcidin, BMP6, SMAD4 and TfR2 expression among groups
Analysis of hepcidin, BMP6, SMAD4 and TfR2 mRNA expression levels among different groups
Analysis of hepcidin, BMP6, SMAD4 and TfR2 mRNA expression levels among different groups
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal