Abstract
Introduction
Pembrolizumab is a humanized monoclonal antibody that disrupts programmed cell death 1 (PD-1) receptor signaling and unmasks native cytotoxic T-cell activity against tumor cells. Early clinical trials show that pembrolizumab induces a prolonged and sometimes dramatic antitumor response in advanced melanoma, renal cell carcinoma, and non-small cell lung cancer (NSCLC) (Topalian et al., 2012; NEJM: 366;26). Consequences of immunomodulation include immunopathologies of skin, endocrine, and/or gastrointestinal organs (Patnaik et al., 2015; Clin Cancer Res: 21;15). Herein we describe the first reported case of Evan's syndrome following the use of pembrolizumab.
Case
A 67-year old man with 70-pack year smoking history presented to hospital with right-sided weakness and gait ataxia. Radiologic studies of head, thorax, abdomen, and limbs revealed left-sided frontal lobe mass, pulmonary lesions, multiple liver metastases, and left lower extremity venous thrombosis. The patient underwent cranial metastasectomy, which confirmed NSCLC primary neoplasm, followed by cranial irradiation, dexamethasone treatment, and indefinite enoxaparin. First and second-line chemotherapy with gemcitabine-carboplatin and pemetrexed failed to control his disease; he was therefore enrolled in the MK 3475-001 clinical trial and received investigational pembrolizumab (10mg/kg every 2 weeks).
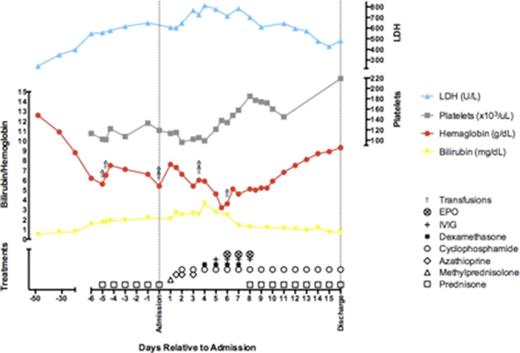
Following 4 cycles of pembrolizumab therapy, repeat CT scans showed a marked reduction in cerebral, liver, and lung tumor burden. Treatment continued with transient interruption of therapy at cycle 8 due to delayed side effects of cranial radiation. An additional 10 cycles (18 cycles total) of therapy were delivered, after which the patient was admitted to hospital for new-onset fatigue, exertional dyspnea, and jaundice. Laboratory evaluation showed a hemoglobin of 6.2 g/dL, reticulocyte count 239 ×103/µL, and platelet count 113 × 103/µL, with elevated total bilirubin of 1 mg/dL, LDH 398 U/L, haptoglobin level less than 20 mg/dL, and normal creatinine and liver enzymes. Peripheral blood film revealed microspherocytes and thrombocytopenia, indirect and direct testing revealed a pan-reactive IgG autoantibody, while serologic testing was negative for infectious and rheumatologic diseases. Diagnosis of warm-autoimmune hemolytic anemia was made and the patient was initiated on prednisone, azathioprine, cyclophosphamide, and IVIG therapy in conjunction with erythropoietin injections and transfusion support. Hemoglobin reached a nadir of 3.2 g/dL before recovering to 11.6 g/dL at 2 weeks into admission, followed by discharge on daily oral prednisone at 105 mg and cyclophosphamide 100 mg.
Sustained hematologic stability permitted gradual prednisone taper to a dose of 20 mg. Fourteen weeks post-admission, the patient returned to hospital with severe bruising and epistaxis. Laboratory evaluation showed hemoglobin of 11.6 g/dL and platelet count of 6 × 109/L, concerning for ITP and fulfilling criteria for Evan's syndrome. Salvage therapy commenced with weekly rituximab and re-initiation of high-dose prednisone, until platelets recovered to 146 × 109/L following the fourth/final dose of rituximab. Prednisone taper was re-trialed with complete discontinuation of steroids after 24 weeks. Despite a persistently positive Coombs test, the patient maintained normal hemoglobin and platelet counts 40 weeks after rituximab therapy. Opportunistic Aspergillus fumigatus pulmonary infection followed salvage therapy, but was successfully treated with voriconazole over 12 weeks. CT staging scans at 48 weeks post-discontinuation of pembrolizumab demonstrated stable metastatic disease.
Conclusion
Reversal of cancer-induced immune suppression through PD-1 inhibition has the potential to provoke immunodysregulation against multiple hematologic lineages. Though autoimmune hyperactivation persists for months after pembrolizumab cessation, immune-related hemolysis and thrombocytopenia remain amenable to treatment with corticosteroids and rituximab. Despite immune downregulation with corticosteroids and rituximab, pembrolizumab appears to exhibit sustained immune control over advanced malignancy in this case.
Off Label Use: Rituximab to treat refractory ITP. Butler:Merck: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal