Abstract
Background
Anti-thymocyte globulin (ATG) is used as prophylaxis against graft-versus-host-disease (GVHD). Dosing regimens for ATG are weight-based and largely empiric. An ideal dose of ATG would prevent severe GVHD yet maintain graft-versus-tumor effect and allow for adequate immune reconstitution to prevent severe infections. Previous studies suggest ATG dose reduction from 7.5 mg/kg to 6 mg/kg is associated with decreased risk of infections (Hamadani et al, Blood, 2009), while further decrease to 2.5 mg/kg is associated with a higher risk of GVHD (Mohty et al, Blood 2003).
The targets of ATG, recipient T-cells post-cytotoxic therapy and T-cells in the stem cell graft, are not a function of recipient weight. Furthermore, current dosing strategies do not account for patient specific factors. We hypothesized the recipient peripheral blood absolute lymphocyte count (ALC) on the first day of ATG administration would interact with the amount of ATG administered to influence transplant outcomes.
Methods
We retrospectively analyzed 135 patients who received rabbit ATG (Thymoglobulin¨) for GVHD prophylaxis after allo-HCT between 1/1/2005 and 12/31/2013. All dosing was based on recipient weight and changes over the 8 years reflect programmatic changes in dosing strategies. Of these, 24 patients (18%) received 10 mg/kg ATG, 43 patients (32%) received 7.5 mg/kg ATG, and 68 patients (50%) received 5 mg/kg ATG. Additional GVHD prophylaxis included calcineurin inhibitor and methotrexate for ablative conditioning and calcineurin inhibitor and mycophenolate mofetil for reduced intensity conditioning. Disease and treatment-related characteristics are summarized in Table 1.
Results
The median follow-up from day of transplant was 26.4 months (range 1.1 to 97.3). The 100-day cumulative incidence of grade II-IV acute GVHD was 20%, 13% and 6% in the in 5 mg/kg, 7.5 mg/kg and 10 mg/kg groups, respectively, p = 0.36. The 2-year cumulative incidence of chronic GVHD in the 3 groups was 57%, 58%, and 52%, respectively, p = 0.88. Severity of chronic GVHD, per NIH consensus criteria, was lower with higher doses of ATG (24% vs. 17% vs 0%, respectively, p = 0.026).
There was no difference in the 2-year overall survival for patients who received 5 mg/kg, 7.5 mg/kg, or 10 mg/kg (60% vs 51% vs 46%, respectively, p = 0.39). However, infectious complications were significantly higher with higher doses of ATG (1% vs 9% vs 17%, p = 0.026). Similarly, invasive fungal infections increased with higher doses of ATG (0% vs. 0% vs. 8%, p = 0.009).
The median total amount of ATG given was 568 mg (range 127.7 to 1342.6). Multivariate analyses using the cox-proportional hazard model showed no significant impact of total amount of ATG (HR= 1.024, p = 0.12) or CIBMTR disease risk (HR= 1.65, p = 0.06) on overall survival.
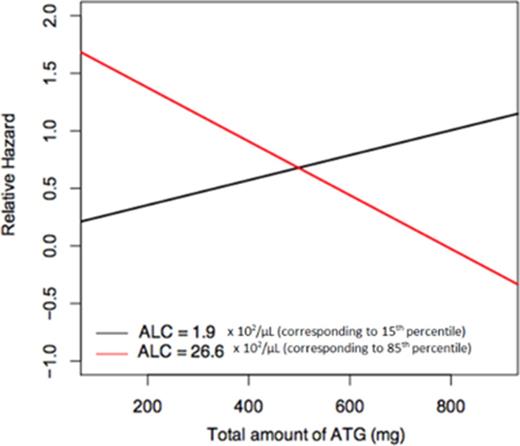
The median peripheral blood ALC on the day of ATG administration was 11.5 x 102/μL (range 0.0 - 58.14). The interaction of total ATG and ALC was a favorable predictor for overall survival (HR= 1.12, p = 0.05). For ALC at the 15th percentile (1.9 x 102/μL), increasing total ATG was associated with higher risk of death. For ALC at the 85th percentile (26.6 x 102/μL), increasing total ATG was associated with lower risk of death (Figure 1).
Conclusions
Our study validates previous findings that increasing dose of ATG increases risk of infection. Novel findings show that decreasing dose of ATG increases severity of chronic GVHD. In addition, we show the recipient ALC on the day of transplant interacts significantly with the total amount of ATG and influences overall survival. Future studies to optimize ATG dosing based on ALC are warranted to further improve the therapeutic window of ATG.
| Variable . | 5 mg/kg . | 7.5 mg/kg . | 10 mg/kg . |
|---|---|---|---|
| Regimen | |||
| Reduced-Intensity | 63% | 74% | 100% |
| Ablative | 37% | 26% | 0% |
| Donor | |||
| Matched | 96% | 86% | 100% |
| Mismatched | 4% | 14% | 0% |
| Stem Cell Source | |||
| Peripheral Blood | 91% | 70% | 83% |
| Bone Marrow | 9% | 30% | 17% |
| Primary Disease | |||
| Acute Leukemia | 38% | 37% | 41% |
| Myeloid Disorder | 44% | 17% | 21% |
| Lymphoid Disorder | 17% | 23% | 38% |
| Other | 1% | 23% | 0% |
| Variable . | 5 mg/kg . | 7.5 mg/kg . | 10 mg/kg . |
|---|---|---|---|
| Regimen | |||
| Reduced-Intensity | 63% | 74% | 100% |
| Ablative | 37% | 26% | 0% |
| Donor | |||
| Matched | 96% | 86% | 100% |
| Mismatched | 4% | 14% | 0% |
| Stem Cell Source | |||
| Peripheral Blood | 91% | 70% | 83% |
| Bone Marrow | 9% | 30% | 17% |
| Primary Disease | |||
| Acute Leukemia | 38% | 37% | 41% |
| Myeloid Disorder | 44% | 17% | 21% |
| Lymphoid Disorder | 17% | 23% | 38% |
| Other | 1% | 23% | 0% |
Off Label Use: Antithymocyte globulin for prophylaxis for graft-versus-host disease..
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal