Abstract
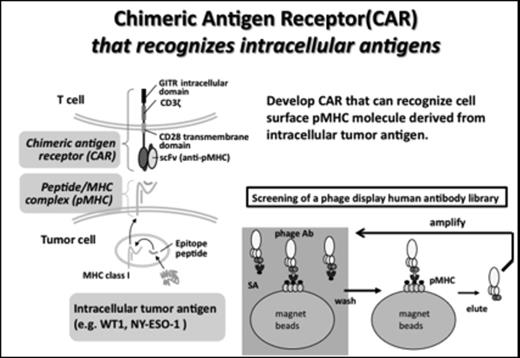
Adoptive cell therapy with lymphocytes transduced with chimeric antigen receptor (CAR) is a promising strategy to treat cancer patients. Recent success in the treatment of patients with B cell malignancy by CD19-CAR encourages the development of successive CAR therapy targeting other tumor-associated antigens. However, the search for the appropriate target molecule for CAR, other than B cell markers, is a serious question. The target of CAR is generally limited to the cellular surface molecules, making difficult to expand CAR therapy for broad range of cancer patients. Inspired by the physiological recognition of epitope peptide and MHC molecule (pMHC) by T cells, we have generated a series of antibodies that recognize the pMHC complexes with peptides derived from tumor antigens expressed intracellularly.
We isolated an scFv antibody clone WT#213 that can specifically recognize WT1 p235-243 peptide (CMTWNQMNL) complexed with HLA-A*24:02 molecule by the screening of human antibody scFv phage display library. We have constructed retrovirus that encodes the CAR consists of WT#213 and intracellular signal transduction domains of CD3z and GITR (WT#213 CAR). We confirmed the specific recognition of endogenous WT1-expressing cells by the CAR-T cells with the intracellular cytokine staining and the 51Cr release cytotoxic assay. Utilizing NOG immunodeficient mice, we demonstrated the effectiveness of adoptive cell therapy with WT#213 CAR against the WT1 expressing HLA-A*24:02 positive human leukemia cells.
To evaluate the safety of the WT#213 CAR, we predicted the potential property of WT#213 CAR to cross-react to normal tissues in humans. We conducted alanine scan analysis of WT1p235-243 peptide that was recognized by WT#213 CAR as well as the TCR derived from CTL clone TAK-1 which recognizes same epitope peptide in association with HLA-A*24:02 to define the amino acids that were critically important in the recognition by the WT#213 CAR or TAK-1-derived TCR. After BLAST search, we synthesized the normal protein-derived peptides with potential risk of cross-reactivity, and tested the recognition of these peptides by WT#213 CAR or TAK-1-derived TCR. Although the critical peptides, and therefore the peptides with potential risk, were quite different between the WT#213 CAR and TAK-1-derived TCR, none of these peptides showed the stimulation of WT#213 CAR or TAK-1-derived TCR.
The results here suggest that the immunotherapy with WT#213 CAR will be effective for the treatment of the leukemia patients without the predicted risk at least in the evaluation we performed.
Ikeda:Takara Bio Inc.: Research Funding. Akahori:Takara Bio Inc.: Research Funding. Miyahara:Takara Bio Inc.: Research Funding. Amaishi:Takaa Bio Inc.: Employment. Okamoto:Takara Bio Inc.: Employment. Mineno:Takara Bio Inc.: Employment. Takesako:TAKARA BIO INC.: Employment. Fujiwara:Celgene: Honoraria, Other: Travel, Acomodations, Expenses.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal