Abstract
Background: Venous thromboembolism (VTE) is one of the most common complications of patients with brain tumors. There is limited data available in the literature on VTE treatment in these patients compared to other cancer types. We evaluated the efficacy and safety of low-molecular weight heparin treatment for newly diagnosed VTE in patients with primary and metastatic brain tumours at a tertiary care centre.
Methods: We conducted a matched retrospective cohort study of patients with primary or metastatic brain cancer who were diagnosed with cancer-associated VTE. Cases were selected after completion of a retrospective chart review of consecutive patients who were diagnosed with cancer-associated VTE between January 2010 and January 2014 at the Juravinski Thrombosis Clinic, Hamilton, Ontario, Canada. Controls were age- and gender-matched patients with cancer-associated VTE from the same cohort, but without brain tumours. The primary outcome was first recurrent VTE and secondary outcomes were major bleeding and clinically relevant bleeding.
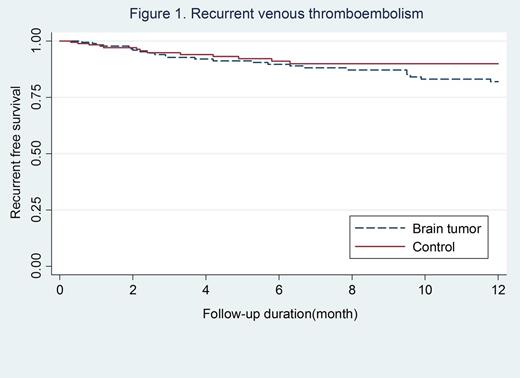
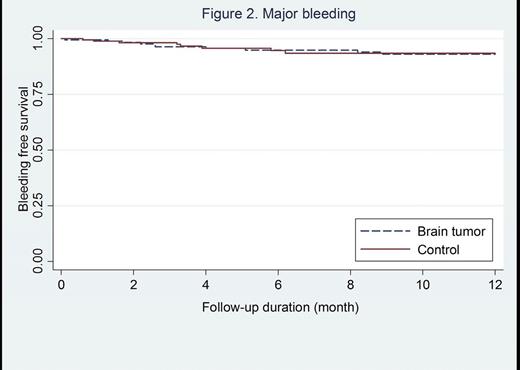
Results: A total of 364 patients with cancer-associated thrombosis were included (182 with primary or metastatic brain tumors and 182 controls). The median follow-up duration was 6.7 (inter quartile range 2.5-15.8) months. The incidence rate of recurrent VTE was 11.0 per 100 patient-year (95% confidence interval [CI]; 6.7-17.9) in patients with brain tumors and 13.5 per 100 patient-year (95% CI; 9.3-19.7) in controls, incidence rate ratio [IRR]; 0.8 (95% CI; 0.4-1.5, p-value=0.43). There was no significant difference in the rate of recurrent VTE in the two groups (log-rank p-value=0.26, Figure 1). The incidence of major bleeding was 8.9 per 100 (95% CI; 5.2-15.4) patient-year in patients with brain tumors versus 6.0 per 100 patient-year (95% CI; 3.4-10.9) in controls, IRR; 0.8 (95% CI; 0.4-1.5, p-value=0.51). There were no significant differences in the risk of major bleeding (Figure 2) and clinical relevant bleeding between the two groups, log-rank p-value 0.9 and 0.8, respectively. When compared to controls, the rate of major gastrointestinal bleeding was lower in patients with brain tumours (0.6% versus 6.0%, p-value=0.003) whereas the rate of intracranial bleeding was higher (4.4% versus 0%, p-value=0.004). Subgroup analysis revealed that the incidence of intracranial bleeding in patients with primary brain tumors was higher than those with metastatic brain tumors, but did not reach statistical significant (6.0% vs 3.5%, p=0.008).
Conclusions: Recurrent VTE, major bleeding and clinical relevant bleeding were not significantly different in patients with cancer-associated VTE in the setting of primary or metastatic brain tumours compared with controls. However, intracranial bleedings occurred more frequently in patients with brain tumours.
Linkins:Pfizer: Honoraria; Bayer: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal