Abstract
Background
Bortezomib (Velcade®) is an inhibitor of the 26S proteasome used as a first line therapy for multiple myeloma, for retreatment of relapsed multiple myeloma and for treatment of AL amyloidosis. An increased incidence of reactivation of herpesviruses including varicella zoster virus (VZV) and herpes simplex virus type 1 (HSV-1) has been reported following administration of bortezomib (Oakervee et al 2005) In addition one study has described an increased incidence of cytomegalovirus (CMV) reactivation in myeloma patients post autografting treated previously with bortezomib when compared to other regimens (Marchesi et al 2014). We noted several cases of CMV viraemia with one case of likely end organ involvement (colitis) among patients given bortezomib. To determine how frequently this association occurs, we introduced a clinical protocol to monitor our patients on bortezomib for CMV infection.
Methods
All patients with myeloma or amyloidosis due their first dose of bortezomib were tested for CMV by polymerase chain reaction (PCR) on whole blood at baseline prior to treatment and then tested every 2 weeks after their initial dose or whenever they re-attended for clinic appointments, regardless of symptoms. Where possible, data on pre- treatment CMV serostatus was also recorded.
We applied a protocol for pre-emptive therapy that we use for transplant patients (Atabani et al 2012). Whereas transplant patients with a viral load greater than 3000 genomes/ml (2520 IU/ml) whole blood are treated, bortezomib patients who had CMV levels in excess of 7500 copies/ml were given treatment (either oral valganciclovir or IV ganciclovir) until 2 consecutive blood samples showed undetectable levels.
Results
60 new bortezomib starters were tested with 43 being tested by CMV PCR at baseline i.e pre-treatment. The characteristics of these patients are shown in table 1
Patient characteristics
| Characteristic . | . |
|---|---|
| Sex | Male = 35 Female = 25 |
| Age | Median 63.5 (range 33-89) |
| Underlying disease | Amyloidosis =39 Multiple myeloma= 21 |
| Pre-treatment CMV serostatus | IgG positive = 35 IgG negative =17 Unknown= 8 |
| Characteristic . | . |
|---|---|
| Sex | Male = 35 Female = 25 |
| Age | Median 63.5 (range 33-89) |
| Underlying disease | Amyloidosis =39 Multiple myeloma= 21 |
| Pre-treatment CMV serostatus | IgG positive = 35 IgG negative =17 Unknown= 8 |
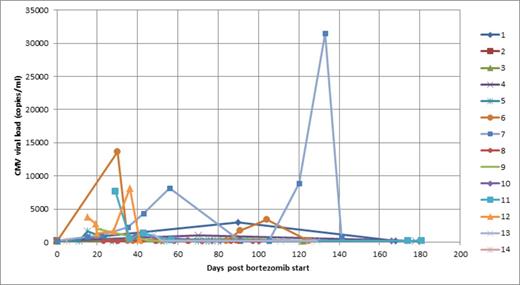
CMV viraemia was detected in 14/60 (23%) patients (10 myeloma and 4 amyloidosis) of whom 13 were known to be seropositive showing that the majority of the viraemias seen were reactivations/reinfections rather than primary infections. No CMV viraemia was seen in the seronegative group. Of those who had a true baseline none were positive prior to starting treatment. Of those without a true baseline, 5 had a detectable viraemia on their first blood sample. The course of the viraemia in these 14 patients is shown in figure 1. Five patients (all myeloma) reached the threshold for treatment and all responded to therapy including one patient who had 2 separate CMV reactivations. There were no cases of CMV end-organ disease.
Summary
In our cohort, 14/60 (23%) patients developed a CMV viraemia after starting bortezomib, corresponding to 13/35 (37%) who were initially CMV seropositive. Although we cannot exclude CMV reinfection from an exogenous source, we suggest that most of these were reactivations of latent CMV. Clinically, the majority of these episodes were self limiting although, because we elected to treat those patients who reached a pre-defined threshold of 7500 copies/ml, the true course of viraemia and the risk of subsequent end organ disease is unclear. Further investigation of the incidence of CMV reactivation and its clinical significance is warranted.
Course of viraemia in 14 patients with detectable CMV following bortezomib.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal