Abstract
Background: Many patient (pt) and disease factors have been shown to impact prognosis in multiple myeloma (MM). A recently described frailty scale categorized pts with MM into 3 groups: fit, intermediate, and frail based on age, comorbidities, and physical and cognitive functioning (Palumbo, Blood, 2015). These groups were predictive of risk of death. The pivotal phase 3 FIRST trial examined the impact of continuous therapy with lenalidomide plus low-dose dexamethasone until disease progression (Rd continuous) on outcomes in transplant-ineligible pts with newly diagnosed MM (NDMM; Benboubker, N Engl J Med, 2014). In this study, Rd continuous resulted in progression-free survival (PFS) and overall survival (OS) benefits compared with melphalan-prednisone-thalidomide (MPT). This analysis examined outcomes in pts by severity of frailty score in the FIRST trial, the largest clinical trial of transplant-ineligible NDMM to date.
Methods: Pts with NDMM who were ineligible for transplant were randomized to treatment with Rd continuous, Rd for 18 cycles (Rd18; 72 weeks), or MPT for 12 cycles (72 weeks). Pts were categorized into 3 severity groups using a score based on age, Charlson Comorbidity Index score, and the Activities of Daily Living (measures of self-care) and Instrumental Activities of Daily Living (measures of usual activities) scales from the EQ-5D questionnaire at baseline, as previously described (Palumbo, Blood, 2015). This analysis examined PFS and OS outcomes by severity group in the overall pt population and between the Rd continuous and MPT arms, which were the primary comparators in this study, using an updated data cutoff of March 3, 2014.
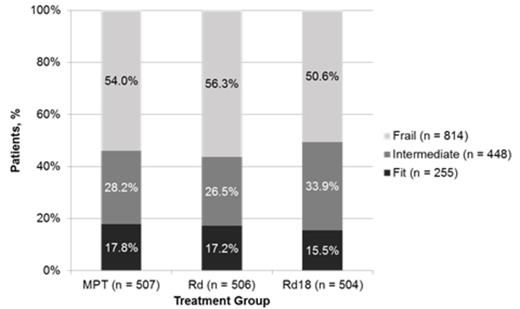
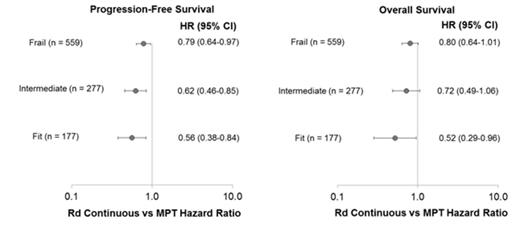
Results: This analysis included 1517 of 1623 pts from the intent-to-treat population; 106 pts were excluded because they did not have the required baseline data from the EQ-5D. The majority of pts enrolled in the FIRST trial were frail (54%) compared with fit (17%) or intermediate (30%). Similar breakdowns were observed across treatment arms (Figure 1). Frail pts were older and had higher Eastern Cooperative Oncology Group performance status scores, higher International Staging System (ISS) stage, higher lactate dehydrogenase levels, and worse renal function than fit or intermediate pts. In the overall pt population, better fitness was associated with significantly improved OS compared with worse fitness: fit vs intermediate (hazard ratio [HR] = 0.66; P = .004), fit vs frail (HR = 0.42; P < .0001), intermediate vs frail (HR = 0.62; P < .0001). Rd continuous treatment prolonged PFS compared with MPT for all pt severity groups (Figure 2). Rd continuous reduced the risk of progression or death vs MPT by 44%, 38%, and 21% in fit, intermediate, and frail groups, respectively. OS was also extended with Rd continuous vs MPT for all pt severity groups (Figure 2). Risk of death was reduced by 48%, 28%, and 20% in the fit, intermediate, and frail groups, respectively, with Rd continuous compared with MPT. Causes of death were primarily MM or complications of MM in fit (62% of deaths) and intermediate (57% of deaths) pts; while they were the cause of death in half of frail pts. Pts were further categorized within each fitness group by ISS stage: fit pts were classified by stage I vs II/III and intermediate and frail pts were classified by stage I/II vs III. Addition of ISS stage further improved prognostic classification within each severity group for both PFS and OS.
Discussion: Results validated the feasibility of using the frailty scale for predicting risk of death in pts with NDMM and further demonstrated an association with PFS outcomes. The majority of pts fell into the frail category, which was associated with the poorest outcomes, indicating a need to further improve outcomes for a large proportion of pts with MM. Rd continuous resulted in PFS and OS benefits compared with MPT regardless of severity of frailty level, with the greatest benefits observed in fit pts. Poor outcomes of frail pts are likely further impacted by a higher proportion of deaths due to causes independent of MM compared with fit and intermediate pts, such as pre-existing comorbidities. These data support the use of Rd continuous as a standard frontline treatment option, even for frail pts with NDMM who are older and may have comorbidities or physical or cognitive impairments.
Facon:Celgene: Membership on an entity's Board of Directors or advisory committees; Jansen: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; Onyx: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Hulin:Celgene Corporation: Honoraria; Bristol Myers Squibb: Honoraria; Amgen: Honoraria; Janssen: Honoraria. Dimopoulos:Janssen-Cilag: Honoraria; Genesis: Honoraria; Amgen: Honoraria; Genesis: Honoraria; Janssen: Honoraria; Onyx: Honoraria; Janssen-Cilag: Honoraria; Celgene: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Novartis: Honoraria. Mohty:Celgene Corporation: Honoraria, Research Funding. Zamagni:Celgene Corporation: Honoraria, Speakers Bureau; Janssen Pharmaceuticals: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Renwick:Amgen: Other: Travel; Bayer: Speakers Bureau. Tempescul:Gilead: Other: Export Board Committee. Palumbo:Novartis, Sanofi Aventis: Honoraria; Celgene, Millennium Pharmaceuticals, Amgen, Bristol-Myers Squibb, Genmab, Janssen-Cilag, Onyx Pharmaceuticals: Consultancy, Honoraria. Sturniolo:Celgene Corporation: Employment. Ervin-Haynes:Celgene Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal