Abstract
Background
Carfilzomib (CFZ), an epoxyketone proteasome inhibitor, was approved in July 2012 for the treatment of patients with relapsed and refractory multiple myeloma (RRMM) in the US. The approved dosing schedule then (before a new label released in July 2015) was an intravenous infusion on days 1, 2, 8, 9, 15, and 16 of a 28-day cycle. The starting dose is 20 mg/m2/day during Cycle 1; if well tolerated, it should be escalated to a target dose of 27 mg/m2/day in Cycles 2+. There exists evidence in oncology studies that reductions from standard dose and dose-intensity may compromise health outcomes (Lyman GH 2009); however, literature based on real-world CFZ dosing data is sparse. The objective of this study was to retrospectively assess the association between CFZ dose escalation in routine clinical practice and duration of therapy (DOT).
Methods
Data were obtained from a large US-based electronic medical record database, which comprises about 20% of US cancer patients who are geographically and demographically diverse. Adult multiple myeloma (MM) patients were included if they were: receiving CFZ between July 20, 2012 and April 30, 2015 after previous exposure to bortezomib and an immunomodulatory agent; no CFZ use before MM diagnosis; >1 cycle of CFZ use. To adjust for clinical practice variations, 10% variability was allowed for the recommended dose levels of 20 mg/m2 and 27 mg/m2. Patients with all their Cycle 1 doses within 10% of 20 mg/m2 were included in an analysis of dose escalation. Patients were classified as "early escalators" if their dose increased to 27 mg/m2 on day 29 (start of Cycle 2), or as "late escalators" if the dose increased to 27 mg/m2 after day 29, or as "non-escalators" if their dose never increased to 27 mg/m2 during the follow-up. DOT was calculated from CFZ initiation date to the last day of that CFZ regimen (ended by treatment discontinuation, death or loss to follow up, whichever comes first). The effect of dosing escalation ("early escalators" vs. "late escalator" vs. "non-escalator") with regard to DOT was assessed using Kaplan-Meier method; statistical significance of differences in DOT across three groups was evaluated with Cox proportional hazards regression model, controlling for patient's baseline demographic and clinical characteristics and also occurrence of events (e.g., renal complications, cardiac events, hematologic comorbidities) in Cycle 1.
Results
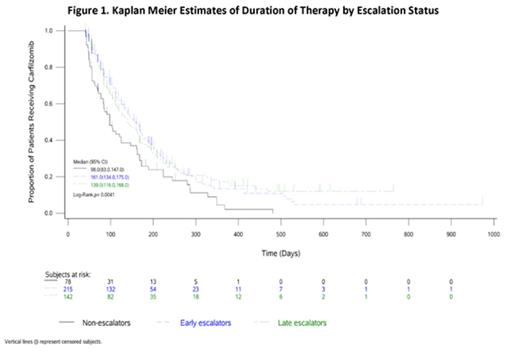
A total of 435 CFZ users meeting selection criteria were retrospectively identified, 54% male, with a median (interquartile range [IQR]) age of 69 (61-76) years and 65% (n=283) aged 65+; 70% were White, 16% Black, 2% Asian and 12% Other. More than half (57%, n=248) lived in the South, 18% in the Northeast, 13% in the Midwest, and 12% in the West. In the 1-year before CFZ start, 40% of the patients had chronic kidney disease, 18% had kidney failure, and 2% had a cardiac condition; at CFZ initiation, 14%, 31%, 8% and 2% of patients had ECOG performance status of 0, 1, 2, 3, respectively. During the course of CFZ treatment (i.e., DOT, median 112 days, IQR 70-196 days), 15% had a concomitant use of granulocyte-colony stimulating factor (G-CSF); 49% (n=215) escalated to ≥27 mg/m2 at the start of Cycle 2, 33% (n=142) escalated later than Cycle 2 start, and the remaining 18% (n=78) did not escalate. Among the two escalator groups, median (IQR) time to first escalation was 28 (28-28) days for "early escalator" and 35 (30-42) days for "late escalator"; both were within Cycle 2 (days 29-56) as standard dose escalation. Median DOTs were 161 days for "early escalator", 139 days for "late escalator" and 98 days for "non-escalator", p=0.0041 (Figure 1). Multivariate Cox proportional hazards modeling showed that early and late escalators had 34% (hazard ratio [HR]=0.66, p=0.002) and 38% (HR=0.62, p=0.001) lower risks of CFZ discontinuation relative to non-escalators, respectively. In addition, with (vs. without) concomitant G-CSF use suggested a 25% (HR=0.75, p=0.047) lower risk to discontinue CFZ.
Conclusions
The data from this study indicate that CFZ administration in standard dose escalation is associated with lower risk of treatment discontinuation for RRMM patients. Further investigation of the time-varying effect of dose escalation as well as treatment-induced adverse events is needed to understand the impact of various factors on physician's decisions in clinical practice to continue or discontinue multiple myeloma treatment.
Harvey:Onyx/Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Chen:Onyx Pharmaceuticals: Employment. Lethen:Amgen: Employment. Mahue:Amgen - Onyx Pharmaceuticals: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal