Abstract
Recently, a striking example of the effects of acquired somatic mutations in splicing factors such as SF3B1, U2AF1, and SRSF2 has been described. The DNA sequences from abnormal blood cells of patients with hematologic malignancies has shown that a high proportion of these diseases are associated with somatic mutations of spliceosomal proteins. However, little is known about the aberrant splicing pathways in multiple myeloma (MM) patients. Therefore, this study investigated the presence and prognostic implication of splicing gene aberration in MM patients. Also, we planned functional experiments in cultured normal and Hela cell line to determine the effects of the common spliceosome mutations on cellular growth rate, cell count, and apoptosis. In total, 87 MM patients and 100 healthy controls were examined for somatic mutations in SF3B1, U2AF1, and SRSF2 by direct sequencing. Also, the clinical and laboratory characteristics of MM patients were reviewed. Regarding to the initial findings in recent reports, this study expressed the wild-type and the mutant (Q157P) U2AF1 in Hela cells and examined their proliferation.
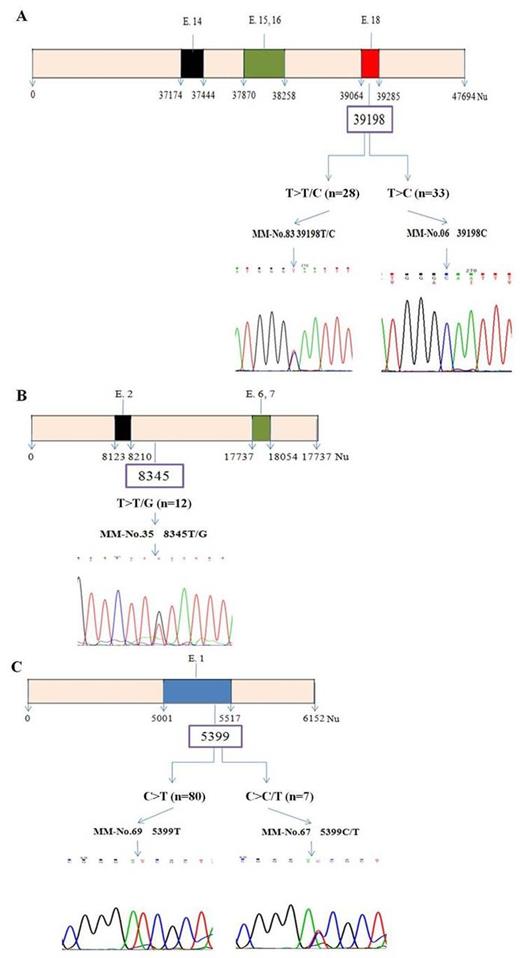
MM patients did not show mutation in SF3B1, U2AF1,and SRSF2 splicing genes. However, the patients displayed 39198T>C or 39198T>T/C polymorphism (70.1 %) in exon 18 of SF3B1, 8345T>T/G polymorphism (13.8 %) in exon 2 of U2AF1, and 5399C>T or 5399C>C/T polymorphism (100.0 %) in exon 1 of SRSF2 (Fig. 1). In the entire cohort, the number of patients with one polymorphism, two polymorphisms, and three polymorphisms was counted up to 24.1 %, 67.8 %, and 8.0 %, respectively. Meanwhile, in 100 normal controls, polymorphism of SF3B1 exon 18 and U2AF1 exon 2 was counted up to 82.0 % and 10.0 %, respectively. Free light chain (FLC) kappa/lambda (K/L) ratio and chromosome analysis showed significant difference according to polymorphism status (p=0.002) while other clinical characteristics did not. The patient with polymorphisms in both SF3B1 and U2AF1 gene had unfavorable overall survival (p =0.067) and disease-free survival (p=0.001), compared to patients without polymorphism.
Our results show no recurrent SF3B1 and U2AF1 mutations in MM patients. Instead, polymorphisms were found in SF3B1 or U2AF1, which were significantly implicated in worse prognosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal