Abstract
BACKGROUND. The most frequently mutated genes documented in blood cells of aging individuals are known epigenetic regulator genes such as TET2 and DNMT3A, which suggests that alteration of epigenetic homeostasis could be a predisposing factor in the pathogenesis of several age-associated hematological malignancies. This study is aimed at determining if changes in hematopoietic 5-hydroxymethylcytosine (5hmC) and 5-methylcytosine (5mC) levels occur in normal individuals, and if they are independent of acquired mutations in epigenetic regulators.
METHOD. The study population comprised 198 unrelated women randomly selected to form four age categories (neonates, 25-30 years, 70-75 years and >90 years) from the general community. Genomic DNA from total blood cells or cord blood (12.5 ug) was hydrolysed using DNA Degradase Plus and analysed by mass spectrometry (LC-ESI-MS/MS-MRM) to quantify global 5-methyl-2'-deoxycytidine and 5-hydroxymethyl-2'-deoxycytidine levels. Statistical analysis (normality test, outlier detection, descriptive statistic, non-parametric Kruskal-Wallis and Mann Whitney tests) were performed using NCSS 07.1.21 to correlate methylation (5mC and 5hmC) levels with i) X-chromosome inactivation (XCI) patterns (evaluated by HUMARA) in polymorphonuclear (PMN) and ii) telomeres length (measured by the method of Cawthon). All individuals over 70 were sequenced in search for mutations in epigenetic regulator genes including TET2, DNMT3A, ASXL1, IDH1, IDH2 and WT1.
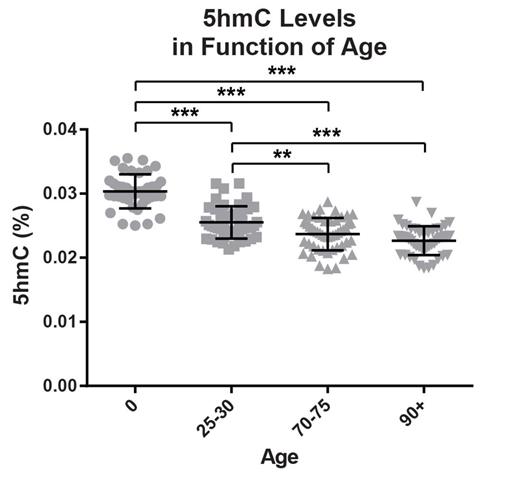
RESULTS. 5hmC and 5mC levels: Global 5hmC levels decline steadily with age in human blood cells (30% from birth to old age, P <0.000001). This reduction is progressive between 0 and 75 years of age and plateaued at a low level thereafter (Figure 1). A less severe reduction in 5mC level was also observed between newborn and elderly individuals (3%, P<0.000024). Correlation with biological characteristics: Low level of 5hmC was associated with more important XCI skewing in PMN (age-adjusted, P =0.0304) as well as a reduction of telomere length (age-adjusted, P =0.0354), both surrogate markers of clonal dominance. Correlation with mutational status: Of the 100 individuals over 70 years, 16 had a somatic mutation in TET2, 10 in DNMT3A and none in IDH1, IDH2 or WT1. Individuals with TET2 gene mutation (variant allele frequency (VAF) >10%) had a significant reduction in 5hmC but not the DNMT3A subjects. To evaluate if the TET2 mutated individuals were the sole drivers of the documented reduction in 5hmC, we removed these subjects from the statistical analysis and correlation was preserved. However, correlation with XCI skewing and telomere reduction was no longer significant.
CONCLUSION. These results document a significant age-associated reduction in 5hmC during aging in the hematopoietic compartment. DNA hypohydroxymethylation should be considered as a new hallmark of aging hematopoiesis. We also demonstrate that acquired mutations in key epigenetic regulators such as TET2 are not the sole cause of this reduction. Epigenetic drift, which is defined as the cumulative selection of permissive stochastic epigenetic modifications, is responsible for most of the age-associated loss. These results also indicate that the impact on clonal dominance may be different according to the cause of 5hmC (mutation vs drift). Further studies are needed to evaluate the impact of 5hmC reduction on the risk of hematological cancer initiation.
Busque:Novartis: Consultancy; BMS: Consultancy; PFIZER: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal