Abstract
Background
Tyrosine kinase inhibitors (TKIs) are the standard of care for pts with CP-CML. Current recommendation is to continue TKI therapy indefinitely but previous studies indicate that pts with deep and sustained molecular responses (MRs) on imatinib (IM) may achieve long-lasting TFR. Nilotinib (NIL) at 300mg BID induces higher rates of deep MRs compared to IM and high dose NIL (400mg BID) enables a substantial proportion of pts who do not obtain MR4 (BCR-ABL1IS £ 0.01%) or MR4.5 (BCR-ABL1IS £ 0.0032%) with IM to reach such deep MRs levels, potentially compatible with TFR. However, optimal duration of treatment with NIL to ensure the highest rate of TFR after treatment discontinuation is unknown.
Objective
ENESTPath was designed to assess the optimal duration of NIL therapy that is necessary to achieve and maintain TFR upon treatment discontinuation in pts pretreated with IM.
Methods
ENESTPath is a randomized, phase III study enrolling CP-CML pts who after at least 2 years (yrs) of IM therapy achieved a complete cytogenetic response (CCyR), but not yet a MR4. After enrollment, pts were assigned to receive NIL at 300 mg BID for 2 yrs or 3 yrs (Arm 1 and Arm 2, respectively). Patients who will obtain a stable MR4 or better for at least 12 months (mo) will enter the TFR phase. Primary endpoint is to evaluate the proportion of pts in both arms who will remain in TFR for ≥1 yr after NIL discontinuation.
Results
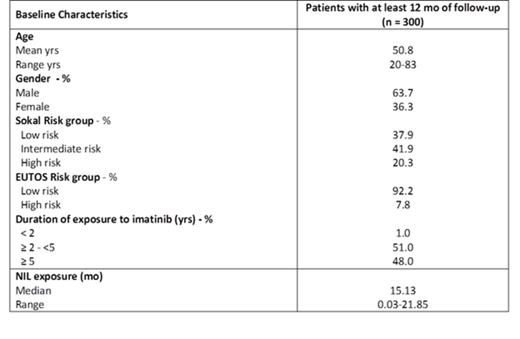
620 pts were enrolled in the study between May-2013 & Apr-2015. In this interim analysis, the first 300 pts (mean age 50.8 yrs; 63.7% male) enrolled and treated with NIL for ≥1 yr have been included. Baseline characteristics are detailed in the Table.
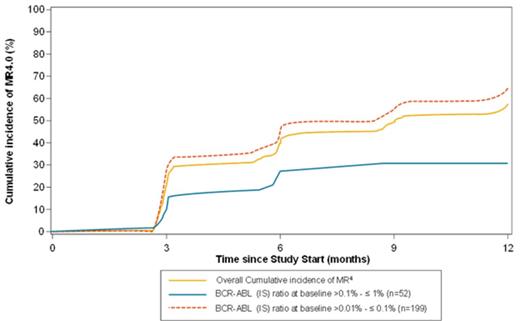
By 12 mo of NIL treatment, cumulative incidences of newly acquired MR4 and MR4.5 were 57.4% and 30.5%, respectively.
Further analysis of MR4 achievement showed that pts with a major molecular response (MMR: BCR-ABL1IS >0.01% - ≤0.1%) at baseline had a higher probability to achieve a MR4 than those lacking MMR at baseline, with a cumulative incidence of MR4 by 12 months of 64.8% and 30.8%, respectively (Figure).
Adverse events (AEs) were mostly of grade 1-2, manageable with supportive care or NIL dose interruption/reduction and included pruritus (19%), headache (9%), skin rash (9%), upper abdominal pain (8%) and constipation (7%).
Grade 3-4 hematologic AEs were uncommon. The incidence of grade 3-4 laboratory abnormalities was low: lipase increase, hyperglycemia, ALT and AST increase, hyperbilirubinemia and hypercholesterolemia reported in 3.7%, 1.3%, 1%, 0.7%, 0.3%, 0.3% pts, respectively.
Grade 3-4 ischemic cardiovascular events were experienced by 5% of pts including peripheral artery occlusive disease (1.7%) and coronary artery disease (3.7%) (1 pt experienced both AEs).
Sub-analyses aiming to evaluate the impact of baseline SCOREa CV risk factor on the onset of arterial ischemic events are currently ongoing. Results on 168 pts showed grade 3-4 ischemic CV events in 19% of pts who were at very high or high risk (n = 47) compared to 1.7% of in pts with moderate or low risk (n = 121).
During the first 12 mo, 48 (16%) pts discontinued NIL therapy: 32 discontinued due to AEs/laboratory abnormalities, 12 withdrew consent, 4 due to other reasons (protocol deviation, pregnancy and non-compliance). No patients left the study due to progression to AP/BP. Till date there were no on-treatment deaths.
Conclusions
This interim analysis shows that a switch to NIL at lower doses than in prior studies (300mg BID instead of 400mg BID) induces high rates of MR4 and MR4.5 in pts without such MR levels on IM. The safety profile of NIL at 300mg BID is consistent with that described in other prospective studies.Thus a switch to NIL for pts not achieving a deep MR during IM therapy is predicted to substantially increase the probability of achieving TFR requirements. A longer follow-up is necessary to assess what may be the best duration of NIL prior to treatment discontinuation.
aRisk factors evaluated by applying the SCORE chart proposed by the European Society of Cardiology
Rea:Novartis: Honoraria; BMS: Honoraria; Ariad: Honoraria; Pfizer: Honoraria. Rosti:Bristol Myers Squibb: Honoraria, Research Funding, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau. Cross:Qiagen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Ariad: Consultancy, Honoraria, Research Funding. Hellman:Novartis: Research Funding; BMS: Research Funding. Niederwieser:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Almeida:Shire: Speakers Bureau; Bristol Meyer Squibb: Speakers Bureau; Novartis: Consultancy; Celgene: Consultancy. Dezzani:Novartis: Employment. Pellegrino:Novartis: Employment. Costantini:Novartis: Employment. Walasek:Novartis: Employment. Saglio:Bristol-Myers Squibb: Consultancy, Honoraria; Novartis Pharmaceutical Corporation: Consultancy, Honoraria; ARIAD: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Steegmann:Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Ariad: Honoraria, Research Funding. Baccarani:NOVARTIS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ARIAD Pharmaceuticals, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal