Abstract
Introduction: Nilotinib is a second-generation TKI that exhibits significant efficacy as first- or second-line treatment in patients with CML. Superior rates of deeper molecular responses were achieved with nilotinib vs. imatinib in patients newly diagnosed with CML in chronic phase in the ENESTnd trial. In addition, the 24-month analysis of the ENESTcmr study demonstrated that switching to nilotinib after a minimum of 2 years on imatinib led to increased rates of MR4.5 by 24-month vs. remaining on imatinib (42.9% on nilotinib vs. 20.8% on imatinib). Although our CMR study revealed that ABCG2 421C/A genotype was an independent predictor of CMR in imatinib-treated CML patients (Shinohara et al, Haematologica, 2013), a relationship between ABCG2 421C/Agenotypes and CMR rate has not been observed in CML-CP patients treated with Nilotinib until now. The purpose of this study is to evaluate the efficacy of nilotinib in patients with CML-CP who have achieved a MMR with imatinib. In addition, the correlation of deeper molecular responses with clinical, immunological, and pharmacokinetic parameters in CML-CP patients was evaluated.
Methods: Patients with CML-CP who had achieved MMR but still had persistent BCR-ABL positivity by RQ-PCR on imatinib were eligible. Nilotinib will be taken twice daily (600 mg/day) for 2 years. Thirty-five institutions in STAT study group participated. The study was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was written by all patients according to institutional guidelines. The study was approved by all institutional review boards and registered with ClinicalTrials.gov (number UMIN000005903). The primary objective of this multicenter phase II, single arm, open-label clinical study was to identify the MR4.5 rate at 24 months after the initiation of nilotinib treatment. The molecular response was evaluated according to the International Scale RQ-PCR upon study entry and every 3 months thereafter. Nilotinib trough concentrations were determined using high-performance liquid chromatography.Genotyping of SLC22A1 (156T>C, 480G>C, 1022C>T, and 1222A>G), ABCB1 3435T>C, ABCG2 421C>A, and the *6, *27 and *28 alleles of UGT1A1 were performed using a polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. Flow cytometry-based analysis of peripheral blood T cell subsets was performed using a whole blood lysis technique.
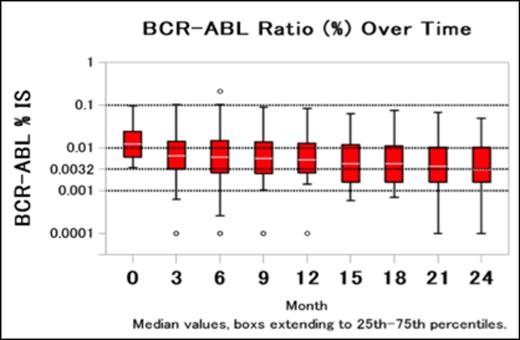
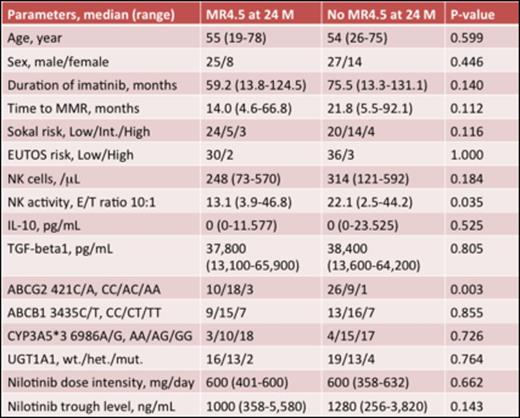
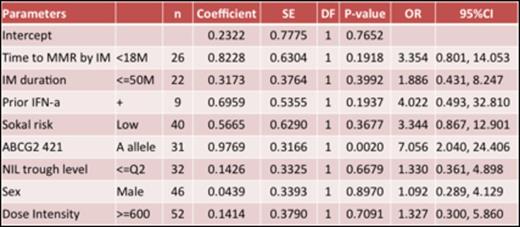
Results: Between July 2011 and December 2012, 80 Japanese patients were recruited, and 74 patients were evaluated in the study. The median age was 54.5 years. The ratio of men to women was 52:22. The median duration of imatinib treatment was 62.5 months (range, 13.3-131.1 months). All patients showed MMR at the time of entry into the study and the median time to MMR on imatinib therapy was 20.2 months (range, 4.6-92.1 months). The median duration of treatment was 17.9 months (range, 0.5-25.1 months) for nilotinib 300 mg twice daily. Dose reduction and interruption were seen 17.1% and 18.4% in safety data set (N=76), respectively. The patients achieving deeper molecular responses increased over time (Figure1). The rates of MR4 in the evaluable patients were 69% and 73% at 12 and 24 months, respectively, and the rates of MR4.5 were 28% and 49% at 12 and 24 months, respective. Comparison of the clinical backgrounds, pharmacokinetic, pharmacogenomic, and immunological parameters (NK cells, NK activity, and cytokines) in patients with MR4.5 at 24 months (n=33) and without (n=41) is performed. Age, sex, Sokal risk score, prior IFN therapy, duration of imatinib treatment and time to MMR were not significant predictive factors for achieving MR4.5 at 24 months. The mean nilotinib plasma trough concentration was not significantly different in both groups either (Table1). However, the frequency of patients with ABCG2 421A allele was significantly higher in patients with MR4.5 than without (OR 7.056, 95%CI 2.040-24.406, P =0.002). This finding suggested that the ABCG2 421A allele was associated with a higher intracellular retention and therefore, higher nilotinib exposure than the wild-type genotype.
Conclusion: We confirmed that switching to nilotinib led to deeper molecular responses in CML patients with persistent residual disease on long-term imatinib therapy.
Nakaseko:Otsuka: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Research Funding, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau. Takahashi:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Honoraria, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Masis: Consultancy; Astellas: Speakers Bureau; Sysmex: Research Funding, Speakers Bureau; Celgene: Speakers Bureau; Otsuka: Membership on an entity's Board of Directors or advisory committees. Nishiwaki:Novartis: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal