Abstract
Introduction: Ibrutinib, a covalent inhibitor of Bruton's tyrosine kinase (BTK), is clinically established as an effective treatment for chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL). Further, recent trials reported that ibrutinib combined with anti-CD20 monoclonal antibodies (CD20 mAbs) led to overall response rates (ORRs) of 95% and 100% in relapsed CLL patients (Burger et al Lancet Oncol 2014, Jaglowski et al Blood 2015), and an ORR of 87% in relapsed MCL patients (Wang et al ASH 2014), indicating complementary therapeutic effects from the 2 drugs. However, some preclinical studies showed that ibrutinib inhibited antibody-dependent cell-mediated cytotoxicity and phagocytosis (ADCC and ADCP). This discrepancy may be due to differences between experimental conditions (eg, drugs used at micromolar concentrations for sustained periods of time) relative to the pharmacokinetics (PK) of ibrutinib, which has a typical peak plasma concentration (Cmax) of ~200 nM 2 hours (tmax) after oral administration of 420-560 mg, and is cleared rapidly from circulation (Davis et al ASH 2014; Advani et al J Clin Oncol 2013). Using clinical PK as a basis, we designed in vitro and in vivo studies to re-evaluate the impact of ibrutinib on ADCC and ADCP.
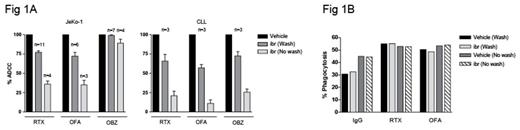
Methods: To model the effect of covalent inhibition by ibrutinib long after tmax, peripheral blood mononuclear cells (PBMC) from multiple healthy donors were incubated for 2 hrs with 200 nM ibrutinib, washed, and rested for 12 hours to represent a time halfway to the next daily dose. Natural killer (NK) cells purified from these PBMC were cultured with JeKo-1 (MCL line) or primary CLL cells opsonized with the CD20 mAbs rituximab (RTX), ofatumumab (OFA), or obinutuzumab (OBZ; washout protocol). To assess the effect of ibrutinib at tmax, treated PBMC, without being washed or rested, were used to purify NK cells for an ADCC assay in the presence of 200 nM ibrutinib (no wash protocol). Dead target tumor cells were counted by flow cytometry and presented as % maximum killing by NK cells from PBMC treated with vehicle. In ADCP assays, % PBMC-derived macrophages that had phagocytosed target cells are shown. In a xenograft model of MCL, SCID mice (10/group) bearing subcutaneous Mino tumors were treated with CD20 mAbs with or without daily oral dosing with ibrutinib at 12 mg/kg. Tumor size was recorded twice weekly. Plasma concentration of ibrutinib was measured using LC-MS. BTK occupancy in tumor cells by ibrutinib was determined as described previously (Dubovsky et al Blood 2013).
Results: The ADCC activity of NK cells treated with ibrutinib rebounded significantly after the washout procedure. Killing of RTX-opsonized JeKo-1 and primary CLL cells rose from 36% and 21% (no wash) to 77% and 71% (wash), respectively. Similar results were obtained using OFA. The killing of OBZ-opsonized JeKo-1 cells was not substantially affected with or without washouts (Fig 1A). Phagocytosis was not affected in the presence of 200 nM ibrutinib (Fig 1B). In tumor-bearing mice, ibrutinib administered at 12 mg/kg by oral gavage resulted in a Cmax of 338 ng/mL (767 nM), exceeding the Cmax observed in patients receiving up to 560 mg a day. However, total drug exposure over time (AUC0-last = 391 ng·h/mL) was within range (Advani et al J Clin Oncol 2013, de Jong et al Cancer Chem Pharmacol 2015). In contrast to previous preclinical study results, ibrutinib significantly enhanced the therapeutic effect of CD20 mAbs (Fig 2). At study termination, we confirmed 98.4 ± 0.3% BTK occupancy in tumor cells.
Conclusions: We recommend ibrutinib concentrations used in cellular assays and in vivo models reflect pharmacologic levels of ibrutinib in patients administered 420-560 mg once daily. The optimal dose in animal studies can be determined by PK/PD measurements. Our data indicate that NK-mediated ADCC recovers significantly 12 hours after ibrutinib has been removed, which may be attributed to turnover of ITK, or other signaling components of ADCC that are targets of ibrutinib during the rest period. ADCP activity of macrophages was not affected by 200 nM ibrutinib. These data suggest that the 2 major effector cell types for tumor-targeting antibodies are not as inhibited by clinically relevant concentrations of ibrutinib as have been described previously. This is supported by the present in vivo study data showing that ibrutinib did not inhibit, but augmented the therapeutic effect of CD20 mAbs.
Ng:Pharmacyclics LLC, an AbbVie Company: Employment. Lu:Pharmacyclics LLC, an AbbVie Company: Employment. Sukbuntherng:Pharmacyclics LLC, an AbbVie Company: Employment. Neuenburg:Pharmacyclics LLC, an AbbVie Company: Employment. James:Pharmacyclics LLC, an AbbVie Company: Employment. Chang:Pharmacyclics LLC, an AbbVie Company: Employment.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal