Abstract
Background: Pre-clinical studies have shown that host immune system activation by G-CSF/GM-CSF enhances the biologic activity of rituximab through up-regulation of CD11b/18 expression on neutrophils, increase in the number of circulating granulocytes and higher CD20 epitope density on tumor cells. To further explore the role of augmenting neutrophil function in B-cell lymphoma, we conducted an open-label, single-arm, phase II study evaluating the safety and clinical efficacy of peg-filgrastim and rituximab in patients with low-grade CD20 positive B-cell Non-Hodgkin lymphomas (B-NHL) [chronic lymphocytic leukemia (CLL), follicular lymphoma (FL) and marginal zone lymphoma (MZL)]. (NCT01682044)
Materials and methods: Twenty patients with untreated or relapsed/refractory indolent lymphoma were treated with rituximab (375 mg/m2) every other week for 4 doses, and after 8 weeks every 2 months for 4 additional doses (total 8 doses). Peg-filgrastim (6mg) was administered subcutaneously 3 days before each dose of rituximab. Clinical responses and tolerability were examined according to standard international criteria. Biologic monitoring included evaluation of phenotype characteristics of host neutrophils, changes in oxidative burst, and in vitro functional assays including CMC and ADCC at baseline and before each dose of rituximab.
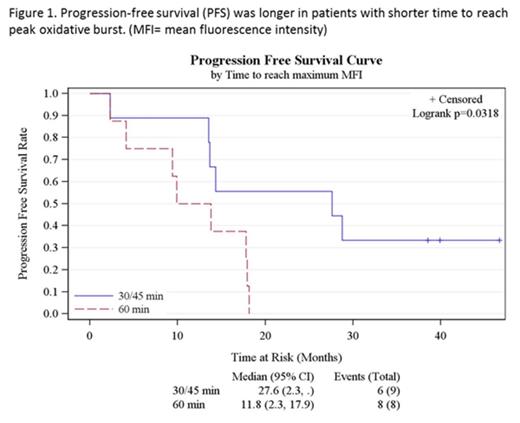
Results: Baseline patient characteristics were as follows: Median age: 64 (28-86) yrs; 70% male; FL: 70%, CLL: 20%, MZL: 10%; Ann-Arbor stage- II- 10%, III- 30% and IV- 60%; disease status- newly diagnosed: 1, rituximab refractory: 2, relapsed ≥1 line of therapies: 17. Median number of prior therapies was 2 (1-5); 75% had previously received rituximab and 90% had received prior anti-CD20 monoclonal antibody therapy. The addition of peg-filgrastim to rituximab was well tolerated, and did not increase the frequency or severity of rituximab-related toxicities. Most of the adverse events were grade 1-2 infusion-related toxicities. One patient developed grade 4 hyperuricemia. One patient with MZL died while on treatment due to disease progression. The overall response rate (ORR) was 60% (12/20) with a complete response (CR) rate of 35% (7/20). Median progression-free survival (PFS) was 17.9 months (95% CI- 9.9-27.6 months); median overall survival (OS) was not reached. A shorter time-to-peak oxidative burst after first dose of peg-filgrastim was associated with higher rates of CR (p= 0.04) and longer PFS (p=0.03, figure 1). Though intensity of peak oxidative burst did not correlate statistically with outcomes, patients who achieved CR (and OR) had consistently higher free radical levels compared to those who did not, especially during the first 2 months of treatment. This trend waned during the latter part of therapy suggesting neutrophil exhaustion. Further analyses are ongoing to evaluate the association between rituximab ex vivo immunological assays and clinical endpoints outcomes.
Conclusion: The combination of peg-filgrastim and rituximab was well tolerated with favorable response rates and PFS compared to historical controls treated with single-agent rituximab. Augmented neutrophil function was associated with higher CR rates and OS. Our results support further evaluation of strategies that enhance the innate immune system to improve rituximab activity in B-cell lymphoma.
Off Label Use: Pegfilgrastim has been combined with rituximab in this phase 2 study for treatment of indolent B-cell Non-Hodgkin lymphoma.. Czuczman:Celgene: Employment; Immunogen: Other: Advisory board; MorphoSys: Consultancy; Boehringer-Ingelheim: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal