Abstract
Background:
Modern therapies incorporating bortezomib (V), lenalidomide (R), cyclophosphamide (C) and dexamethasone (D) constitute the most common doublet (RD, VD) or triplet (VRD, CVD) initial induction regimens for transplant eligible MM in the US. These regimens produce an overall response rate of >80% but their impact on longer term post-AHCT outcomes are largely unknown.
Patient and Methods: We evaluated the relative impact of pre- and post-AHCT treatment on 693 patients receiving upfront AHCT after induction with RD (178), VD (161), CVD (84) or VRD (270) using data prospectively reported to the CIBMTR from 2008-2013. Analysis was limited to those receiving one line of induction chemotherapy and a single transplant with 200 mg/m2 melphalan as conditioning regimen no later than 12 months from treatment initiation. Survival endpoints were evaluated from time of AHCT and the planned use of post-AHCT maintenance incorporating R or V was also considered.
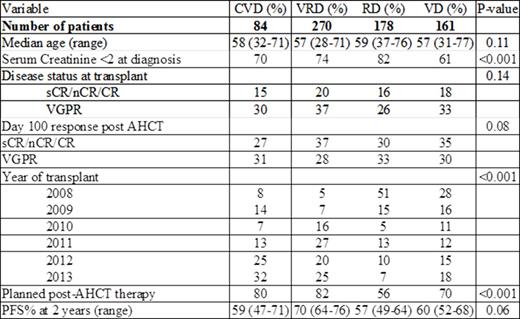
Results: The table shows patient characteristics. Median number of induction cycles were 4 (range, 2 to 16) and median time to transplant was 6.5 months. Median follow up was 36 months (3 to 82) from time of transplant. Age, disease stage, disease status at transplant and cytogenetic risk were similar between the 4 cohorts. Fourteen percent had high-risk chromosomal abnormalities [17pdel, t(4;14), t(14;16), chromosome 1 abnormalities and hypodiploidy]. CVD and VD were used more frequently in patients with renal failure. Doublet use was more common before 2010 (55%) and triplets after 2010 (85%). Use of R or V post-AHCT chemotherapy was higher in with VRD (79%) and CVD (81%) cohorts vs. RD (53%) and VD (67%).
No differences in pre- or post-AHCT responses were seen with regard to choice of induction agents. Pre-AHCT responses ≥VGPR were 57% for VRD vs. 45/42/51% for CVD/RD/VD respectively. Corresponding post AHCT responses at day 100 were 65% for VRD and 58/63/65% respectively.
In multivariate analysis of relapse, progression free (PFS) and overall survival (OS), there was no overall difference in these outcomes based on induction regimen. High risk cytogenetics (HR = 0.57, p=0.0004 for non-high risk) and absence of planned maintenance (HR=1.55, p=0.0008) were associated with higher risk of relapse. VRD was associated with a marginal benefit in relapse risk vs. CVD (HR=0.68, p=0.04). Non-relapse mortality was similar across cohorts. Those not receiving planned post-AHCT maintenance (HR 1.69, p<0.001) and high-risk MM had higher risk of progression/death (HR in non-high risk 0.58, p<0.001); OS was lower in those with low stage (DSS or ISS I/II) vs. stage III (HR 0.6, p=0.006) and with non-high risk cytogenetics (HR 0.5, p=0.001).
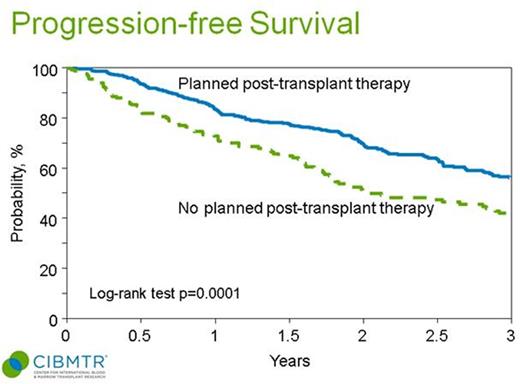
Patients receiving planned post-AHCT therapy had significantly improved 3-year PFS vs. no post-AHCT therapy (55% vs. 39%, log-rank p=0.0001) (figure).
Conclusions:
Modern induction doublets and triplets induce similar response rates and post AHCT outcomes at a median follow-up of 36 mo. Although there is an increase in use of triplets after 2010 in transplant eligible patients, our analysis suggests that the choice of induction regimen is less important than the decision to use vs. not use planned post-AHCT maintenance therapy.
Patient characteristics.
PFS by planned post-AHCT therapy vs. not:
Krishnan:Janssen: Consultancy; BMS: Consultancy; Jazz: Consultancy; Millenium: Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Onyx: Speakers Bureau. Gasparetto:Millennium: Honoraria, Other: Export Board Committee, Speakers Bureau; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Hari:Celgene: Consultancy; Takeda: Consultancy; BMS: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Spectrum: Consultancy; Sanofi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal