Abstract
Introduction
Programmed death ligand 1 (PD-L1) is expressed both on neoplastic cells and on tumor-infiltrating non-neoplastic cells in several types of lymphoma. The programmed death-1 (PD-1)/PD-L1 pathway inhibits antitumor immunity. However, little is known about the functions of this pathway in adult T-cell lymphoma/leukemia (ATLL). The purpose of this study was to investigate the association between clinicopathological features and PD-L1 expression in ATLL.
Methods
We reviewed 134 biopsy samples diagnosed as ATLL including 61 cases in the international peripheral T-cell lymphoma study and 73 cases submitted to the Department of Pathology at Kurume University for consultations.
PD-L1 immunohistochemical staining (IHC) was performed on formalin fixed paraffin embedded samples. According to staining patterns of PD-L1 IHC, we categorized cases of ATLL into several groups. First, cases with ATLL cells with distinct and circumferential membranous and/or cytoplasmic staining of PD-L1 were defined as neoplastic PD-L1 positive ATLL (nPD-L1(+) ATLL) whereas cases without such staining pattern of PD-L1 in ATLL cells were classified as neoplastic PD-L1 negative ATLL (nPD-L1(-) ATLL). Second, among nPD-L1(-) ATLL group, cases with non-ATLL cells with PD-L1 positivity were defined as microenvironmental PD-L1 positive ATLL (miPD-L1(+) ATLL) whereas cases without PD-L1 positive non-ATLL cells were difined as PD-L1 negative ATLL (PD-L1(-) ATLL).
Clinical features analyzed in this study were age at the diagnosis, sex, Shimoyama classification, Eastern Cooperative Oncology Group performance status, LDH, presence or absence of hypercalcemia, Ann Arbor stage, Japan Clinical Oncology Group prognostic index, and complete response (CR) or CR uncertain as therapeutic effect. Morphological variant, and immunohistochemical expression in neoplastic cells of PD-1, CD30, CCR4, and FoxP3 were included in pathological features. The numbers of PD-1 positive tumor-infiltrating lymphocytes (TILs) were counted in each sample as quantitative analysis.
Clinicopathological features of the patients were statistically compared using chi-square tests or Fisher's exact tests. Wilcoxon rank sum tests were performed to compare the number of PD-1 positive TIL counts between different groups. The Kaplan-Meier method was used to estimate the overall survival (OS). To compare the survival curves, we performed the log-rank test. Univariate and multivariate analyses were used to evaluate the influence of prognostic factors on the OS by the Cox proportional hazards models.
Results
The percentage of nPD-L1(+) ATLL and nPD-L1(-) ATLL were 7.5% (10/134) and 92.5% (124/134), respectively. Among nPD-L1(-) ATLL, miPD-L1(+) ATLL were 58.2% (78/134) whereas PD-L1(-) ATLL were 34.3% (46/134).
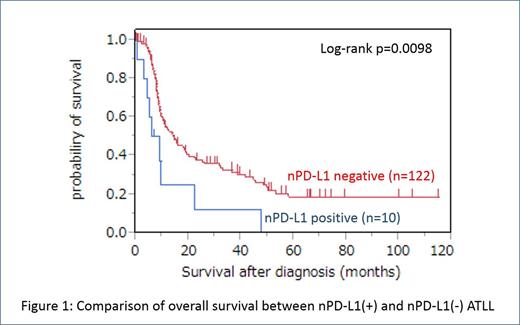
Statistical comparison of clinicopathological features between nPD-L1(+) ATLL and nPD-L1(-) ATLL showed significant difference in overall survival (OS) although significant difference was not observed in other clinicopathological features. NPD-L1(+) ATLL had inferior overall survival (OS) compared with nPD-L1(-) ATLL (p = 0.0098) (Figure1). The PD-L1 expression in neoplastic cells maintained prognostic value for OS in univariate analysis (p = 0.026) and multivariate analysis (p = 0.034).
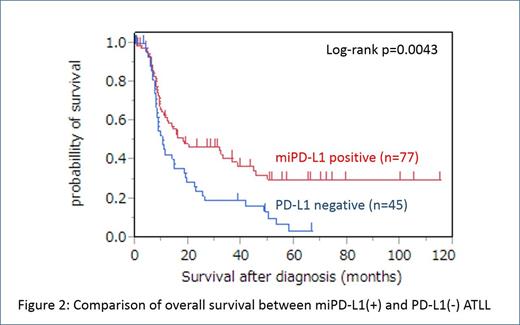
Statistical comparison of clinicopathological features between miPD-L1(+) ATLL and PD-L1(-) ATLL showed statistically significant differences in the positive rate of hypercalcemia (10%, 8/78 in miPD-L1(+) ATLL vs 24%, 11/46 in PD-L1(-) ATLL, p = 0.045), the positive rate of skin lesion (23%, 18/77 in miPD-L1(+) ATLL vs 7%, 3/46 in PD-L1(-) ATLL, p = 0.02), and the number of PD-1 positive TILs (3.60/hpf in average in miPD-L1(+) ATLL vs 0.28/hpf in PD-L1(-), p = 0.0007). MiPD-L1(+) ATLL had superior overall survival (OS) compared with PD-L1(-) ATLL (p = 0.0043) (Figure 2). The expression of PD-L1 in non-ATLL cells maintained prognostic value for OS in univariate analysis (p = 0.0056) and multivariate analysis (p = 0.002).
Conclusion
This study, for the first time, described the impact of PD-L1 expression on clinicopathological features of ATLL. More effective immunotherapy for the PD-1/PD-L1 pathway may be selected according to PD-L1 expression in ATLL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal