Abstract
Background: Treatment of nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is controversial. While management generally follows that of classical HL (cHL), NLPHL demonstrates many biologic differences from cHL, and such patients (pts) are often excluded from prospective trials of novel therapies. The purpose of this study was to describe the characteristics and treatment outcomes of pts with NLPHL at the Princess Margaret Cancer Centre (PM) compared to those with cHL, with both groups managed following the same treatment algorithms according to stage at presentation.
Methods: We identified 820 pts registered in our Lymphoma database from 1999 to 2013 who had primary treatment at PM; 50 had NLPHL and 770 had cHL. The outcomes of the two groups were compared utilizing the whole cohort as well as a 1:3 propensity score-matched subcohort. Propensity scores were calculated based on a logistic regression model with the probability of belonging to the NLPHL group as dependent variable. Pts were matched based on age, gender, treatment received, stage, extranodal disease and follow-up time. A competing risks approach was used to estimate the probability of relapse and Gray's test and Fine and Gray model were used to study significance between groups. A Cox regression model and the log-rank test were used to compare disease free survival (DFS).
Results: Median age at diagnosis of pts with NLPHL was 38 years (range 12-73) and 28 years (4-88) for those with cHL (p=0.006); 20% of NLPHL pts were female versus 44% with cHL (p=0.008). Similar proportions of pts with NLPHL and cHL presented with stage I/II lymphoma (84% vs 73%, p=0.083) but extranodal (E) disease was less common in NLPHL (4% v 25%, p=0.006). Treatment received: NLPHL: radiation (IFRT) alone 26%, chemotherapy alone 20%, combined modality therapy (CMT) 54%; CHL: IFRT alone 2%; chemotherapy alone 24%; CMT 74%. Chemotherapy consisted of doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD). The median follow-up time (range) was 7.7 (1.5-15.2) years in the NLPHL group versus 5.2 (0.03-15.9) years in the cHL group.
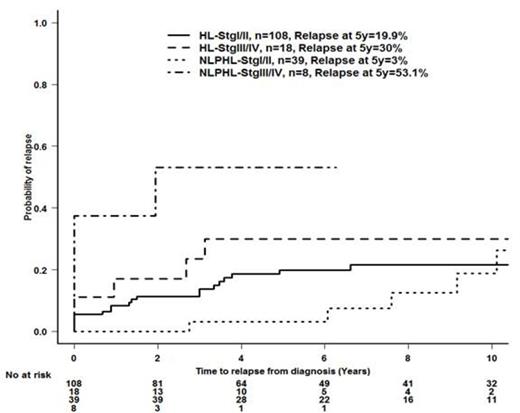
There was no difference in DFS or relapse rate between pts who were diagnosed with cHL and NLPHL (p >0.7). Pts with stage III/IV NLPHL had the poorest outcome with the highest rate of relapse at 5 years (53.1%) compared to other groups (23.4%, 12.4% and 2.8%), and the lowest DFS at 5 years (47% vs. 97%, 85% and 74%, p<0.001). Pts with stage III/IV NLPHL were 2.9 times more likely to experience relapse than pts with stage III/IV cHL (p-0.03 (95% CI: 1.1-7.5)). Among the 50 pts with NLPHL, 47 could be matched with pts with cHL (n=126, Table 1). The response to treatment was similar between the matched pt groups, with 44 (94%) pts achieving CR/CRu/PR in the NLPHL group and 118 (94%) with cHL. As was seen for the whole cohort, no difference in the probability of relapse or DFS between the matched NLPHL and cHL pts (relapse at 5y:11.2% v 21.4%, DFS at 5y: 89 % vs. 75% p=NS); however, the hazard ratio for relapse for pts with stage III/IV NLPHL vs cHL was 3.0 (95% CI 0.83-10.9, p=0.09); relapse probability by subtype and stage is shown in figure 1. DFS at 5 years: NLPHL stage I/II 97%, stage III/IV 47%; cHL stage I/II 78%, stage III/IV 58% ; hazard ratio for DFS for pts with stage III/IV NLPHL vs cHL 2.0 (95% CI 0.53-7.6, p=0.31); Four pts with NLPHL and 1 with cHL developed a second lymphoma (4 DLBCL, 1 plasmablastic).
Conclusion: Within the limits of relatively small patient numbers, the prognosis of pts with early stage NLPHL is excellent when treated with CMT approaches used for cHL, or IFRT alone, while pts with stage III/IV disease appear to have a high rate of recurrence with ABVD; alternative regimens should be considered in these patients.
Characteristics of matched cohorts
| Covariate . | CHL (N=126) N (%) . | NLPHL (N=47) N (%) . |
|---|---|---|
| Age at Diagnosis (yrs) Median (range) | 36 (12-73) | 36 (4-86) |
| Gender % Female Male | 29 (23.0) 97 (77.0) | 10 (21.3) 37 (78.7) |
| Treatment Chemotherapy CMT IFRT | 25 (19.8) 85 (67.5) 16 (12.7) | 10 (21.3) 27 (57.4) 10 (21.3) |
| Stage I/II III/IV | 108 (85.7) 18 (14.3) | 39 (83.0) 8 (17.0) |
| Extranodal N Y | 119(94.4) 7(5.6) | 45 (95.7) 2 (4.3) |
| Response to Treatment CR/CRu/PR NR/SD | 118 (93.7) 8 (6.3) | 44 (93.6) 3 (6.4) |
| Status Alive Death | 110 16 | 45 2 |
| Cause of Death HL or treatment toxicity Other/unknown alive | 6 9 110 | 1 1 45 |
| Competing risk (Relapse) No Relapse Relapse (incl PD) Death without Relapse | 94 25 7 | 38 9 - |
| Covariate . | CHL (N=126) N (%) . | NLPHL (N=47) N (%) . |
|---|---|---|
| Age at Diagnosis (yrs) Median (range) | 36 (12-73) | 36 (4-86) |
| Gender % Female Male | 29 (23.0) 97 (77.0) | 10 (21.3) 37 (78.7) |
| Treatment Chemotherapy CMT IFRT | 25 (19.8) 85 (67.5) 16 (12.7) | 10 (21.3) 27 (57.4) 10 (21.3) |
| Stage I/II III/IV | 108 (85.7) 18 (14.3) | 39 (83.0) 8 (17.0) |
| Extranodal N Y | 119(94.4) 7(5.6) | 45 (95.7) 2 (4.3) |
| Response to Treatment CR/CRu/PR NR/SD | 118 (93.7) 8 (6.3) | 44 (93.6) 3 (6.4) |
| Status Alive Death | 110 16 | 45 2 |
| Cause of Death HL or treatment toxicity Other/unknown alive | 6 9 110 | 1 1 45 |
| Competing risk (Relapse) No Relapse Relapse (incl PD) Death without Relapse | 94 25 7 | 38 9 - |
Kukreti:Roche: Honoraria; Lundbeck: Honoraria; Amgen: Honoraria; Celgene: Honoraria; Janssen Ortho: Honoraria. Kuruvilla:Seattle Genetics: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; Roche Canada: Honoraria. Crump:Celgene: Honoraria; Sanofi: Honoraria; Seattle Genetics: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal