Abstract
BACKGROUND: Alloimmunization to antigens on transfused red blood cells (RBCs) is a major problem in transfusion medicine. Development of alloantibodies limits the availability of compatible RBC units for chronically transfused patients. In addition, chronic RBC transfusion therapy is an important risk factor for cell-mediated bone marrow transplant rejection. To develop anti-RBC alloantibodies, professional antigen presenting cells (APCs) are required to digest and load RBC antigens into MHC class II for presentation to CD4 helper T cells. Mouse studies suggest that RBC-associated minor histocompatibility antigens can also be cross-presented into recipient MHC class I; thereby contributing to CD8 T-cell mediated immune responses against bone marrow allografts. Transfusion of RBCs after prolonged refrigerated storage induces erythrophagocytosis and pro-inflammatory gene expression in the spleen. Splenic red pulp macrophages (RPMs) ingest damaged erythrocytes, catabolize heme, and recycle iron but they are weak APCs. Conversely, dendritic cells (DCs) are the most potent of all APCs. A rare and genetically distinct subset of CD8+ DCs can efficiently ingest dead or dying cells and is uniquely capable of activating CD4 and CD8 T cell responses by presenting antigen via MHC class II and by cross-presenting cell-associated antigens via MHC class I. The RBC storage lesion, in addition to promoting phagocytosis by RPMs, may also induce RBC uptake by splenic DCs. Inflammation induced by stored RBC transfusions, or by antibody-mediated RBC clearance, may enhance DC activation and RBC uptake, thereby triggering humoral and cell-mediated immune responses to RBC antigens.
AIMS: A mouse model of green fluorescent protein (GFP)+ RBC transfusion and flow cytometry were used to track the uptake of fresh, antibody-coated and stored RBCs into splenic professional APC subsets and the effects of these RBCs on APC activation.
METHODS: Cohorts of 8-12 week old C57BL/6 male mice (n=4 per group) were transfused with one mouse-equivalent unit of syngeneic leukoreduced and citrate-phosphate-dextrose-adenine suspended fresh GFP+ RBCs (<24 hours old), stored GFP+ RBCs (11 days old), fresh GFP+ RBCs coated with anti-RBC polyclonal antibodies, or saline. Two hours after transfusion, spleens were dissociated (Liberase™ DL plus DNAse), FcBlocked, and stained with 7-color antibody panels to define CD8+ DCs (CD11c+ MHC-II+ CD8+ DEC205+), CD8- DCs (CD11c+ MHC-II+ CD8-CD11b+), and RPMs (VCAM-1+ CD11blo). Activation markers included MHC class II and CD86. Data were analyzed using FlowJo and Excel.
RESULTS: RBC transfusion after prolonged storage markedly increased GFP+ RBC uptake by RPMs (5.8±0.91% fresh vs. 47±2.3% stored) and CD8+ DCs (1.3±0.27% fresh vs. 13±1.0% stored). A smaller increase in RBC uptake was seen in CD8- DCs (0.6±0.068% fresh vs 1.5±0.083% stored). Antibody-coated RBCs showed two-fold increased uptake by CD8+DCs (25±1.5%) compared with stored RBCs and they showed similar uptake into RPMs (50±1.2%) and CD8- DCs (1.1±0.047%). Lack of GFP+ DC staining with the Ter119 RBC marker confirmed that the GFP signal was internalized and not adsorbed onto the DC surface. CD8+ DCs that internalized GFP+ RBCs showed significantly increased CD86 and MHC class II expression consistent with activation.
CONCLUSION: Taken together, these results suggest that transfusion of stored or antibody-coated RBCs increases RBC uptake by, and activation of, professional splenic APCs. CD8+ DC uptake of stored or antibody-coated RBCs may contribute to humoral and cellular anti-RBC immune responses via antigen presentation in MHC class II and class I, respectively. Thus, these mechanisms may increase the risk of alloimmunization to RBC antigens.
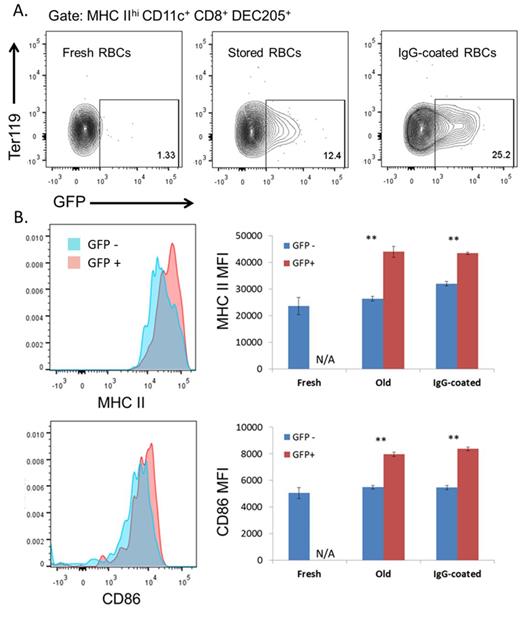
Uptake and activation of CD8+ DCs by transfused old and IgG coated RBCs.
A) Two hours after transfusion of GFP+ fresh, stored and IgG-coated RBCs, spleens were dissociated and APC subsets analyzed using flow cytometry. Representative contour plots show the internalization of GFP+ RBCs by the CD8+ DC population.
B) CD8+ DCs with ingested GFP+ RBCs show increased expression of activation markers. Shown on the left are representative histograms depicting the shift in MHC II (top) and CD86 (bottom) expression in GFP+ (red) compared to GFP- (blue) CD8+ DCs. Shown on the right are the MHC II (top) and CD86 (bottom) median fluorescence intensity (MFI) values (mean ± SEM, n = 4). ∗∗p < 0.01.
Uptake and activation of CD8+ DCs by transfused old and IgG coated RBCs.
A) Two hours after transfusion of GFP+ fresh, stored and IgG-coated RBCs, spleens were dissociated and APC subsets analyzed using flow cytometry. Representative contour plots show the internalization of GFP+ RBCs by the CD8+ DC population.
B) CD8+ DCs with ingested GFP+ RBCs show increased expression of activation markers. Shown on the left are representative histograms depicting the shift in MHC II (top) and CD86 (bottom) expression in GFP+ (red) compared to GFP- (blue) CD8+ DCs. Shown on the right are the MHC II (top) and CD86 (bottom) median fluorescence intensity (MFI) values (mean ± SEM, n = 4). ∗∗p < 0.01.
Zimring:Immucor Inc.: Research Funding; BloodworksNW: Patents & Royalties: Patent Application filed on technology in this abstract - no royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal