Abstract
Background: Inhibitor formation is among the most serious complications of hemophilia A. These alloantibodies are directed against foreign infused factor VIII (FVIII) and occur in up to 25-30% of children with severe hemophilia A, after the first 9-10 exposures to FVIII. The inhibitor neutralizes infused FVIII, requiring alternative "bypass" therapy to treat bleeds, e.g. factor VIIa or FEIBA, to "bypass" the missing factor, and immune tolerance to suppress the inhibitor. Despite these approaches, bleeding is poorly controlled, resulting in 2-fold as many hospitalizations, 10-fold the cost, and 1.7-fold higher mortality. As the burden of disease is high, efforts have been directed at preventing inhibitors. There is increasing evidence that a specialized subset of T cells known as T-regs or CD4+/CD25+/FoxP3+ regulatory T cells, may play a role in mediating immune response to FVIII. Differentiation and function of T-regs are controlled by the X-chromosome-encoded transcription factor, FoxP3, and are generated centrally in the thymus and peripherally in extrathymic tissues. In the inhibitor-prone hemophilia A mouse (FVIII exon 16 knockout) T-regs reduce or prevent immune response to factor VIII: this is presumed to occur through T-reg-mediated hyporesponsiveness of T helper cells to factor VIII. T-regs also mediate maternal tolerance to the fetus in normal pregnancy. In early pregnancy there is an increase in immunosuppressive T-regs, peaking during the second trimester, and declining postpartum. T-regs are hypothesized to modify maternal immune response to the fetal "allograft" to promote tolerance to foreign paternal-derived antigens in the developing fetus. We hypothesized that defects in maternal T regulatory response to factor VIII might be associated with lack of normal FVIII tolerance in their offspring, leading to an alloantibody response, i.e. inhibitor formation. We therefore sought to quantitate and functionally characterize T-regs in mothers of children with congenital hemophilia A with inhibitors, in order to determine if maternal lack of tolerance to FVIII is associated with inhibitor formation in their affected sons.
Methods: Following informed consent, heparinized tubes were drawn from four mothers of hemophilic children, two with and two without inhibitors, cared for at the Hemophilia Center of Western PA (HCWP). T-reg responses to factor VIII were quantitated by flow cytometry, and phenotyping was performed by staining with anti-CD4/Foxp3/CD3/CD25/CD39 monoclonal antibody markers. Functional characteristics of the T-regs were determined in a T-reg proliferation assay using autologous antigen presenting cells (DCs) in the presence or absence of FVIII.
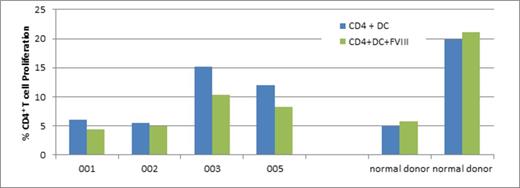
Results: CD4+ T cell proliferative responses to factor VIII (FVIII) were decreased in all four mothers (Figure 1). Mothers of hemophilic sons with inhibitors demonstrated a 1.36-fold decrease (Pt 001) and a 1.1-fold decrease (Pt 002), as compared with mothers of hemophilic sons without inhibitors, who demonstrated a 1.47-fold decrease (Pt 003) and a 1.45-fold decrease (Pt 005) in CD4+ proliferative responses, unlike the two healthy donors who showed minimal (or no) factor VIII response. Patients showed slightly less proliferation with Factor VIII vs. no antigen; healthy donors showed minimal (or no) Factor VIII response (Figure 1).
Discussion: These preliminary results demonstrate lower T cell proliferative responses to FVIII in mothers of children with inhibitor patients than in mother of children without inhibitors. This suggests there is lack of tolerance to FVIII in mothers of children with hemophilia, with or without inhibitors. These responses would also imply these mothers have no tolerance to paternal FVIII, but further studies will be needed to confirm these findings and to characterize maternal T regulatory response to factor VIII, and, in particular, paternal FVIII.
Ragni:Alnylam: Research Funding; Baxalta: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; Biogen: Research Funding; SPARK: Research Funding; Pfizer: Research Funding; Ferring Pharmceuticals: Research Funding; National Hemophilia Foundation: Membership on an entity's Board of Directors or advisory committees; Medscape, Web MD: Honoraria; Genentech Roche: Research Funding; Vascular Medicine Institute: Research Funding; Foundation Women Girls Blood Disorders: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tacere Benitec: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Research Funding; CSL Behring: Research Funding; Dimension Therapeutics: Research Funding; Biomarin: Research Funding. Malec:Biogen: Research Funding; Baxalta: Research Funding. Seaman:Vascular Medicine Institute: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal