Abstract
Background: Chronic ITP in children is an autoimmune disorder characterized by increased platelet destruction and suboptimal platelet production. Pediatric patients with chronic ITP that completed a romiplostim phase 1/2 or phase 3 study could enroll in this open-label long-term extension; 5.2 years of data are reported.
Methods: All patients received SC romiplostim once weekly. The initial dose was the final dose from the parent study or 1 µg/kg (for patients previously receiving placebo or who had not received romiplostim for >24 weeks), adjusted from 1-10 µg/kg to target platelet counts of 50-200×109/L. The incidence of adverse events (AEs) was the 1° endpoint. If approved by investigators, patients who maintained platelet counts ≥50×109/L for ≥4 consecutive weeks at a stable dose could subsequently administer doses by self or caregiver. Patients who turned 18 years of age could remain on study.
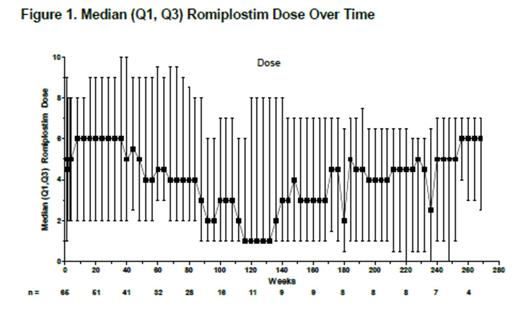
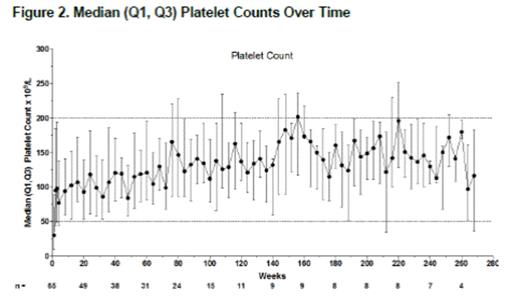
Results: A total of 66 patients (phase 1/2, n=12; phase 3, n=54) entered the extension; 65 received romiplostim for ≤269 weeks (5.2 years); 1 patient withdrew consent before treatment. At baseline, median (range) age was 11 (3-18) years; 56% were female; 61% were white, 14% African American, and 14% Hispanic/Latino; 9.1% had prior splenectomy. Median (range) treatment duration was 57.9 (1-269) weeks; median number of doses was 53.0 (1-268). Median (range) average weekly romiplostim dose was 5.5 (0.1-10.0) µg/kg, including escalation to stable dose, median maximum dose was 8.0 (1.0-10.0) µg/kg, and median most frequent dose was 6.0 (0-10) µg/kg. The median dose fell from 6 to 1 µg/kg; after ~week 140 (n≤9), the median dose fluctuated (Fig 1). Thirteen patients discontinued treatment: consent withdrawn (n=6), noncompliance (2), administrative decision (2), nonresponse (2), and per protocol (1). Fifty-two (79%) patients continued in the study; none withdrew due to AEs. After the first study week median platelet counts remained >50×109/L (Fig 2); for 15 patients (23%), the first study week was the first week receiving romiplostim (ie, previously received placebo). Fifty-six (86%) patients (or caregivers) self-administered romiplostim. Twenty-one (32%) patients received rescue medications on 63 occasions (for low platelet counts [n=35], bleeding/bruising [17], pre- or post-procedure [9], and other [2]), including IVIG (n=10), prednisone (9), aminocaproic acid (3), tranexamic acid (2), methylprednisolone (2), and platelet transfusion (1). Patients required rescue treatment during the first 3 months (27/63 instances), >3-6 months (9), >6-9 months (6), >9-12 months (7), and after 1 year (14) in the extension. Five previous placebo recipients received rescue medication, mostly during the first 3 months (10/14 instances). Three patients achieved remission (platelet counts ≥50×109/L for 24 weeks with no ITP treatments): 1) 9-year-old boy with ITP for 9 years, after 4.3 years of romiplostim, entered remission for the last 2.1 years as of this datacut; 2) 13-year-old boy with ITP for 8.5 years, after 3.3 years of romiplostim, entered remission for the last 1.1 years; and 3) 17-year-old girl with ITP for 8.2 years, after 5.9 years of romiplostim, entered remission for the last 44 weeks. Thirty-four serious AEs occurred in 14 patients, including pyrexia (n=3), epistaxis (2), and thrombocytopenia (2); 3 were deemed treatment-related (anemia, epistaxis, and thrombocytopenia), and none led to discontinuation of romiplostim. Five patients had life-threatening AEs, including thrombocytopenia (n=2) and infection, decreased platelet counts, and subcutaneous abscess (1 each); none were fatal or deemed treatment-related. Bleeding AEs occurred in 47 patients; 3 were deemed treatment-related by the investigator (gingival bleeding, petechiae, and epistaxis). Bleeding AEs occurring in ≥4 patients included contusion (n=25), epistaxis (21), petechiae (16), gingival bleeding (9), mouth hemorrhage (6), ecchymosis (5), and hemorrhage, injection-site bruising, and purpura (4 each). No thrombotic events were reported. There were no peripheral blood abnormalities suggestive of malignancy to warrant a bone marrow examination in any patient. There was no development of anti-TPO or anti-romiplostim antibodies.
Conclusion: In this ongoing open-label extension of children with chronic ITP, romiplostim for ≤5.2 years maintained platelet counts with a safety profile similar to that seen in past studies.
Tarantino:BPL: Membership on an entity's Board of Directors or advisory committees; Grifols: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Octapharma: Membership on an entity's Board of Directors or advisory committees; Baxter: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen, Inc: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Cangene: Research Funding; UptoDate, Inc.: Patents & Royalties: royalties. Off Label Use: Romiplostim is indicated for use in adults with chronic immune thrombocytopenia who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. The use of romiplostim in children with ITP is investigational.. Bussel:Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sysmex: Research Funding; Immunomedics: Membership on an entity's Board of Directors or advisory committees, Research Funding; IgG of America: Research Funding; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen, Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Blanchette:Baxter Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Data Safety Monitoring Board, Research Funding; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Octapharma: Other: Data Safety Monitoring Board; Bayer Healthcare: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Morales:CSL Behring: Membership on an entity's Board of Directors or advisory committees; Baxter: Membership on an entity's Board of Directors or advisory committees. Nie:Amgen Inc.: Employment, Other: Stockholder. Eisen:Amgen Inc: Employment, Other: stock ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal