Abstract
Background We previously demonstrated that Imatinib (IM) could be safely discontinued in patients with undetectable minimal residual disease (UMRD) of at least 2 years (Lancet oncology, 2010;11: 1029-1035). We report the update of the STIM1 study with a median follow-up (FU) of 65 months after IM discontinuation.
Methods In this multicenter STIM study, imatinib (of >3 years duration) was prospectively discontinued in CML pts who were in deep molecular response (DMR) with UMRD sustained for at least 2 years. The UMRD was defined with sensitivity> to 4.5 log with ³ 50,000 ABL transcripts amplified as internal control. Importantly, the molecular relapse (MR) was defined as positivity of BCR-ABL transcript in quantitative RT-PCR confirmed by a second analysis point indicating the increase of one log in relation to the first analysis point, at two successive assessments, or loss of MMR at one point. Quantitative RT-PCR for BCR-ABL transcripts from peripheral blood was performed every month during the first year and every 2 months thereafter. In cases of molecular relapse, the treating physician was recommended to reintroduce TKI treatment. This study is registered with ClinicalTrials.gov, number NCT00478985
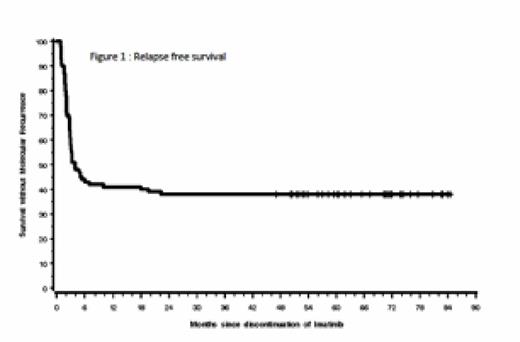
Results From July 9, 2007, to Dec 17, 2009, 100 pts (48 male, 52 female) with CML (50 pts previously treated with interferon) and a median age at inclusion of 59 years (range 29-81) were included in the STIM trial. The median follow-up after treatment discontinuation is 65 months (range 9-84). Five pts died from CML-unrelated causes. Sixty-one pts relapsed of whom 17 have MMR loss at the time of relapse. MR occurred mostly within 6 months after IM discontinuation. Forty-nine (80% of relapsing patients) relapses occurred within month 1 to month 3, 9 (15%) within month 4 to month 7, 3 (5%) within month 18 to month 22. TKI was restarted in 57 out of 61 relapse pts and 55 achieved a second UMRD. With a median FU of 67 months, 39 pts remained free from MR. Among them, 16 were in sustained UMRD while 23 have intermittent positive BCR-ABL assessment without MR. None of the pts experienced a CML progression. The probabilities of relapse-free and treatment-free survivals at 6 months were respectively 43%, 59%; and at 24 months were respectively 38%, 41% after IM discontinuation (figure 1). For those patients who achieved the first 6 months without relapse (landmark analysis) the probability of relapse was 10% at 24 months.
In order to identify factors associated with MR, several baseline characteristic of the study population cohort were analyzed, i.e; gender, age at diagnosis and at IM cessation, Sokal and Eutos risk scores, previous treatment with interferon, time from IM start to first UMRD, duration of UMRD before IM discontinuation and IM duration. Using univariate analysis only age and Sokal score were significant factors linked to MR (p=0.0364 and p=0.0210 respectively). Using multivariate analysis (including Age, gender, Previously IFN and duration of treatment) Sokal risk score was the only variable associated with a significant probability of MR (p: 0.0149 overall and odd ratio 7.422 IC95% :1.251-44.026 p=0.0273 for low versus high risk). With a median FU of 65 months, the cumulative duration of the "off treatment" period of the whole study population was 3113 months.
Conclusion: With a median follow up > 5 years after stopping treatment, STIM study still demonstrates that IM can be safely discontinued in pts with a DMR of at least 2 years duration. MR occurs mostly during the first six months following IM discontinuation and no MR was recorded after 2 yrs. Treatment resumption in relapse patients was associated with the achievement of a second UMRD in almost all patients.
Etienne:BMS: Consultancy, Honoraria; NOVARTIS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; ARIAD: Consultancy, Honoraria; PFIZER: Consultancy, Honoraria. Rea:Novartis: Honoraria; BMS: Honoraria; Ariad: Honoraria; Pfizer: Honoraria. Huguet:Novartis: Consultancy, Research Funding; BMS: Consultancy, Speakers Bureau; ARIAD: Consultancy, Speakers Bureau; PFIZER: Consultancy, Speakers Bureau. Legros:Novartis: Research Funding, Speakers Bureau; BMS: Speakers Bureau; ARIAD: Speakers Bureau. Nicolini:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Ariad Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Rousselot:BMS: Consultancy, Speakers Bureau; ARIAD: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Novartis: Speakers Bureau. Mahon:NOVARTIS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria; ARIAD: Consultancy, Honoraria, Speakers Bureau; PFIZER: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal