Abstract
Introduction: Transient Elastography (TE) of liver is a well established tool to measure liver stiffness, mainly used for assessment of hepatic fibrosis due to chronic hepatitis. Liver biopsy is the gold standard test for measurement of liver iron concentration (LIC) whereas T2* MRI is the best available non-invasive method for the same in thalassemia. We intended to use hepatic TE as an alternative cheaper tool to assess hepatic iron overload so that it can be applied to larger number of patients.
Objective: To assess degree of liver stiffness by TE in patients with HbE beta thalassemia and correlate the findings with LIC calculation by T2* MRI of liver.
Materials and Method: 53 patients with HbE beta thalassemia from the thalassemia clinic of Institute of Haematology and Transfusion Medicine, Medical College, Kolkata were enrolled for the study. Patients with known liver disease were excluded. Baseline data like HbE%, mutations, transfusion requirement, growth status, serum ferritin level etc were collected. All of them underwent TE of liver in the School of Digestive and Liver Diseases, IPGMER using the FibroScan Touch 502 machine (Di Marco et al, British Journal of Haematology, Volume 148,3, 476-479, February 2010). 20 randomly selected patients were also assessed by T2*MRI of liver for hepatic iron assessment at the same time. LIC calculation was done from T2* value (J S Hankins et al, Blood, 14 May 2009, Volume 113:20). Data were analyzed by SPSS software-19, IBM.
Results: The patients with HbE beta thalassemia had a mean HbE level of 53.66 (±18.45) %. Common beta mutations [mostly IVS-1-5(G-C)] usually found in this part of India, were detected. Mean and median age of the study population was 24.11±13.11 years and 20 years, respectively. Median age of 1st transfusion was 11 years. 35.84% patients were non-transfusion dependent. 39/53 patients had facial deformity and growth retardation. Mean baseline hemoglobin was 7.10±0.76 gm/ dl. Mean serum ferritin level was 3183.66±338.45 ng/ml. TE showed 30.18 % patients had severe liver stiffness (Liver stiffness measurement, LSM >15 kPa) whereas 43.34% had minimum stiffness (LSM≤7 kPa). No significant statistical correlation was found between serum ferritin and LSM. 12/20 patients showed very high calculated LIC (>15 mg/g) and lower T2* value (<1.8 ms) whereas only 10% of them showed mildly elevated calculated LIC. Rest had intermediate LIC.
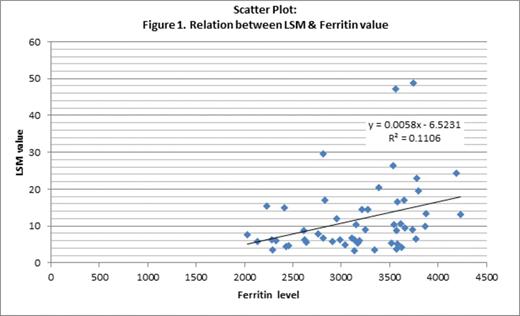
Discussion: There is lack of data regarding hepatic iron overload in HbE beta thalassemia and so also from this part of India. There was a trend that higher the age, higher was the LSM irrespective of the serum ferritin level though not found statistically significant (Figure 1). Serum ferritin level was also not significantly correlated with the calculated LIC in those 20 patients assessed with T2* MRI. 2 patients with mildly elevated LIC had a high ferritin level. Preliminary report indicates that with increase in LSM there was increase in calculated LIC also. Statistical analysis revealed patients with LSM≥7.2 kPa had moderate or severe hepatic iron overload and thus undermine the need for routine T2*MRI. The cut off value signifies that patients with LSM<7.2 kPa might or might not have significantly high liver iron overload, so obviously to be assessed by T2*MRI (Table 1). Therefore use of TE may be an alternative preliminary diagnostic method to gauge hepatic iron overload in HbE beta thalassemia patients. It would be of more value in countries like India where T2* MRI facility is not yet feasible in many centers catering to huge number of HbE-beta thalassemia patients. However, further exploration with larger number of patients is necessary to establish association of LIC and LSM in a more robust way.
Conclusion: In resource-poor countries like India, TE may be a relatively cheap tool to be used as a marker of hepatic iron overload in future.
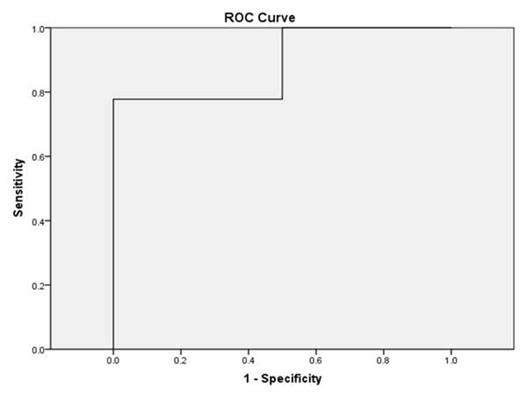
Finding Cut off: ROC (TE-value and LIC categories), n=20
| Positive if Greater Than or Equal Toa . | Sensitivity . | 1 - Specificity . |
|---|---|---|
| 2.3 | 1.00 | 1.00 |
| 3.4 | 1.00 | .50 |
| 4.4 | .94 | .50 |
| 5.7 | .88 | .50 |
| 6.2 | .83 | .50 |
| 6.5 | .77 | .50 |
| 7.2 | .77 | .00 |
| 8.2 | .72 | .00 |
| 8.85 | .66 | .00 |
| 9.45 | .61 | .00 |
| 10.2 | .55 | .00 |
| 11.85 | .50 | .00 |
| 13.85 | .44 | .00 |
| 15.75 | .38 | .00 |
| 18.3 | .33 | .00 |
| 22.9 | .27 | .00 |
| 27.9 | .22 | .00 |
| 35.9 | .16 | .00 |
| 44.7 | .11 | .00 |
| 48.0 | .05 | .00 |
| 49.8 | .00 | .00 |
| Positive if Greater Than or Equal Toa . | Sensitivity . | 1 - Specificity . |
|---|---|---|
| 2.3 | 1.00 | 1.00 |
| 3.4 | 1.00 | .50 |
| 4.4 | .94 | .50 |
| 5.7 | .88 | .50 |
| 6.2 | .83 | .50 |
| 6.5 | .77 | .50 |
| 7.2 | .77 | .00 |
| 8.2 | .72 | .00 |
| 8.85 | .66 | .00 |
| 9.45 | .61 | .00 |
| 10.2 | .55 | .00 |
| 11.85 | .50 | .00 |
| 13.85 | .44 | .00 |
| 15.75 | .38 | .00 |
| 18.3 | .33 | .00 |
| 22.9 | .27 | .00 |
| 27.9 | .22 | .00 |
| 35.9 | .16 | .00 |
| 44.7 | .11 | .00 |
| 48.0 | .05 | .00 |
| 49.8 | .00 | .00 |
Table 2. The smallest cutoff value is the minimum observed test value minus 1, and the largest cutoff value is the maximum observed test value plus 1. LSM more than 7.2 had a sensitivity of 77.2 % and specificity of 100%.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal