Abstract
Red blood cell pyruvate kinase deficiency (PKD) is the most frequent enzyme abnormality of the glycolytic pathway and the most common cause of hereditary nonspherocytic hemolytic anemia. Its incidence has been estimated to be 1 in 20,000, especially in regions where consanguinity is common. It is inherited in autosomal recessive manner occurring as result of homozygous or compound heterozygous mutations affecting both alleles in PKLR gene. Over 200 mutations have been described in patients with PKD causing various severity of hemolysis. Most cases are due to missense mutations, but small deletions, insertions, splice site alterations, frame shifts, disruption of erythroid specific promoters, and nonsense mutations have been reported.
Alu elements are the most abundant mobile DNA sequences in the human genome, contributing to almost 11% of its mass. These mobile elements have contributed significantly to evolution and human diversity by causing gene rearrangements. Alu insertions particularly have been associated with a number of human diseases either by disrupting a coding region or a splice signal. In this abstract, we report an Alu-element insertion in the coding region of PKLR gene as a novel cause of PKD causing severe hereditary nonspherocytic hemolytic anemia.
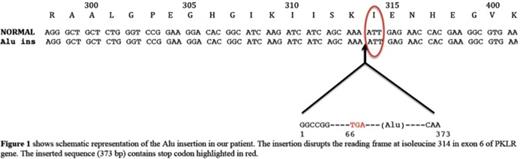
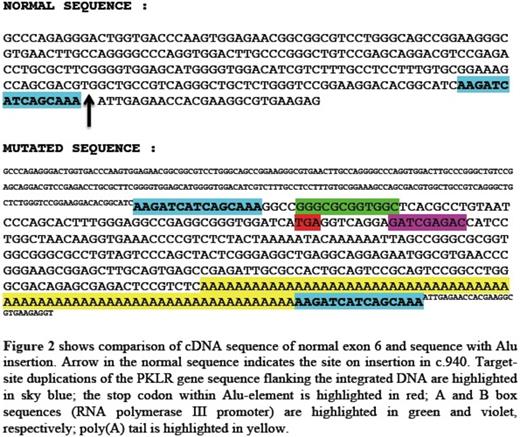
The proband was a 16-month-old middle eastern male born to consanguineous parents. He was diagnosed with severe chronic hemolytic anemia and neonatal jaundice requiring regular blood transfusion since birth. There were no known family members with anemia requiring transfusions. The patient's RBC phenotypic analysis was challenging since most of the circulating RBCs were donor cells. Using a Next-Generation sequencing panel for 27 hemolytic anemia associated genes, we identified and characterized a novel homozygous insertional mutation in exon 6 of PKLR gene along with heterozygosity for ANK1 c.1404+15C>T variant. The mutation in exon 6 was identified as Alu element insertion. The inserted Alu element (~370 bp in size), belonging to the youngest Yb8 subfamily, disrupts the reading frame at isoleucine 314 in exon 6 of PKLR gene, leading to a premature stop codon within the inserted sequence. Quantitative reverse transcription-PCR (qRT-PCR) in the patient's reticulocyte RNA using primers for the sequence before the Alu insertion revealed a transcript present but decreased by 80% compared to the average of controls' reticulocyte RNA. This truncated transcript even if translated is expected to cause severe enzyme deficiency.
Both parents had a normal hemoglobin level with mild reticulocytosis. The mother had a PK enzyme activity of 4.5 units/g Hb (normal range 6.7-14.3 units/g Hb) indicating that she is heterozygous for PKD. Paternal PK activity level was not determined due to insufficient sample. Carrier testing for mutation in PKLR gene in both parents is currently in process at the time of this submission.
The variant in ANK1 gene was predicted to be damaging by affecting the splice site in ankyrin gene. However the father was also heterozygous for this variant and his ektacytometry was normal suggesting a benign nature of ANK1 c.1404+15C>T.
This case represents, to our knowledge, the first report of a pathogenic PKLR mutation due to an Alu element insertion and demonstrates a novel mechanism causing severe hereditary nonspherocytic hemolytic anemia. This report also illustrates the challenge of diagnosing congenital hemolytic anemias in chronically transfused patients. The patients often present with a complex clinical picture and ambiguous laboratory findings and may have several potentially damaging genetics variants identified by next generation sequencing. A critical evaluation of the clinical symptoms, laboratory findings and genetic data from other family members and bioinformatics analysis are required to clarify the contribution of these variants and to arrive at an accurate diagnosis, which will guide the institution of targeted interventions and appropriate genetic counseling.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal