Introduction: Children receiving treatment for cancer commonly experience symptoms during their course that impact patient function. Understanding limitations to patient function caused by symptoms is critical to supporting children through their illness; however assessment of function is not standardized which can result in the under recognition of symptoms. Patient-reported outcome measures (PROs) can systematically measure and quantify the impact of symptoms on patient function. PROs have the potential to improve clinician recognition of symptoms and provide additional objective information about the impact of symptoms on patient function. However, to date there are minimal data using PROs in a clinical setting to detect symptoms and function in children receiving treatment for cancer. The objective of this study was to determine if symptoms causing significant impact on patient function as detected by PROs are recognized by standard clinician interview. We hypothesize that PROs identify symptoms causing significant burden to patients with greater sensitivity than clinician documentation.
Methods: We conducted a prospective study of children age 8-21 years old that began treatment for cancer within the previous 4 weeks. Patients completed self-reported PROMIS questionnaires measuring impaired mobility, pain, fatigue, anxiety, and depressive symptoms monthly at outpatient clinic visits during the first 6 months of cancer treatment. PROMIS is a validated PRO that provides separate scores for each symptom measured with a mean score of 50 and standard deviation of 10. Patients with scores more than 1 standard deviation from the mean (T score ≥ 10 points from mean of 50) have significantly impaired function. Clinician documentation of symptoms at clinic visits were abstracted from the medical record in dichotomous fashion (present/absent) using pre-defined key words to define each symptom. The proportion of clinic visits with impaired function detected by PROMIS was compared to proportion of clinic visits with symptom documented by clinician using chi square tests.
Results: Forty children participated in the study. The mean age of study participants was 11.7 years (SD 4.7). Fifty-eight percent of the patients were male and 60% of patients had leukemia or lymphoma. Paired PRO data/clinician documentation was available for 150 visits. The mean number of clinic visits per study patient was 3.75 (range 2-5).
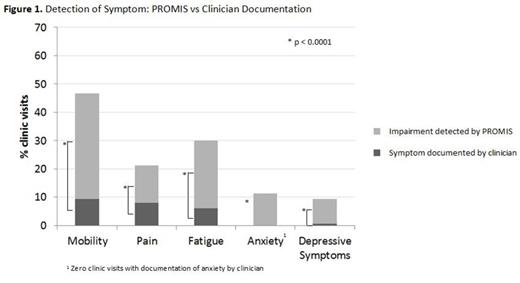
Patients demonstrated impaired function on PROMIS related to any symptom measured at 24% of clinic visits. For all clinic visits at which PROMIS detected impaired function, the symptom was documented by the clinician only 20% of the time (p<0.0001).
Mobility: When impaired function was detected by PROMIS, concerns related to mobility were documented by the clinician only 20% of the time (p<0.0001) (figure 1).
Pain: When impaired function due to pain was detected by PROMIS, pain was documented by the clinician 38% of the time (p<0.0001) (figure 1).
Fatigue: When impaired function due to fatigue was detected by PROMIS, fatigue was documented by the clinician only 20% of the time (p<0.0001) (figure 1).
Anxiety: When impaired function due to anxiety was detected by PROMIS, anxiety was documented by the clinician at 0% of the visits (p<0.0001) (figure 1).
Depressive symptoms: When impaired function due to depressive symptoms were detected by PROMIS, depressive symptoms were documented by the clinician only 7% of the time (p<0.0001) (figure 1).
Conclusion: Symptoms causing significant impact on patient function as detected by PROs are poorly detected and documented by standard clinician interview. These data demonstrate the natural limitations of clinician interview to illicit the true impact of symptoms on patients' lives and the likelihood that clinicians are unaware of the impact of disease and treatment on individuals and their function. The discrepancies in detection of this burden by current practice highlight opportunities to use PRO data to supplement clinical practice.
Panepinto:HRSA, NIH: Research Funding; NKT Therapeutics, Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal