Abstract
Introduction:
High-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (auto-HCT) is the standard of care for younger patients with newly diagnosed multiple myeloma (MM). In approximately 15% of MM patients the monoclonal (M) spike consists of k or l light chains only as opposed to heavy + light chains. It remains unclear whether light chain (LC) MM has a different prognosis compared to other monoclonal protein subtypes after an auto-HCT.
Methods:
We retrospectively analyzed 1067 patients with MM who underwent auto-HCT between January 1, 2004 and January 1, 2011 at our institution. We evaluated the outcome of newly diagnosed patients with LCMM and compared it to patients with IgG or IgA MM, who underwent an auto-HCT after induction therapy. Primary endpoints were complete remission (CR), progression-free survival (PFS) and overall survival (OS) from the date of auto-HCT. Kaplan-Meier analysis with the log-rank test was performed for univariate comparison of survival. Cox proportional hazards regression method was used for univariate and multivariate analyses.
Results:
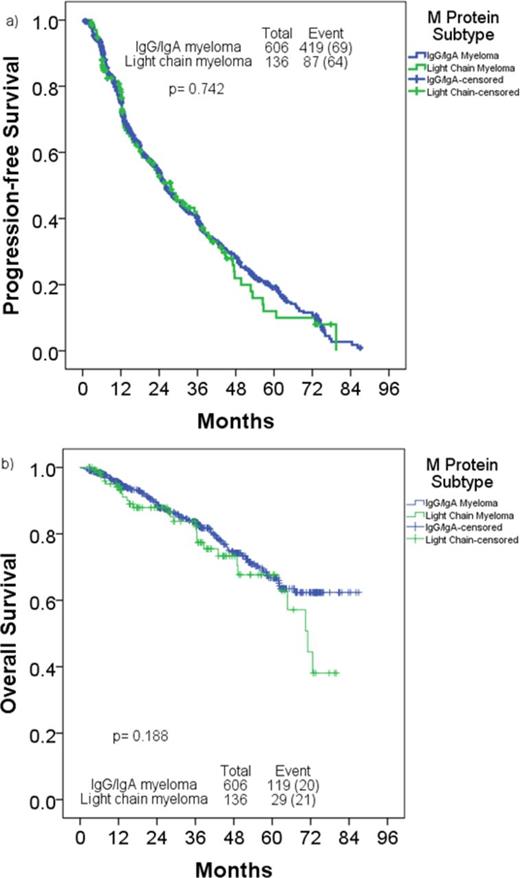
Of 1067 patients who underwent auto-SCT during the period, 223 underwent auto-SCT after relapse, and were excluded. From the remaining 844 who underwent auto-SCT in first remission, we excluded 102 patients (AL amyloidosis 60, POEMS and other plasma cell disorders 10, non-secretory MM 15, IgD 10, IgM 6 and IgE 1) from the analysis. The remaining 742 patients were divided as follows: IgA, 162 patients (22%); IgG, 444 (60%) and LC, 136 (18%). Baseline patient characteristics are described in Table 1. Patients with LCMM were younger and had a higher CR rate to induction. Median follow-up for the entire cohort after auto-HCT was 38 months (range, 0.2-87.0). Post auto-HCT, 28% with IgG/IgA MM and 38% with LCMM achieved a CR (p=0.015). Median PFS was 26.0 months and 27.7 months in IgG/IgA MM and LCMM groups, respectively (p= 0.742). Median OS was not reached and 71.1 months in IgG/IgA MM and LCMM groups, respectively (p= 0.18, Figure 1). Multivariate Cox regression analysis for PFS identified <PR after auto-SCT, non-diploid karyotype, and induction chemotherapy without thalidomide or bortezomib as adverse prognostic factors. Multivariate Cox regression analysis for OS identified presence of hypodiploidy or monosomy 13/del13, higher lactate dehydrogenase pre-transplant, lower hemoglobin pre-transplant, and <PR after auto-HCT as adverse prognostic factors. M protein subtype did not affect PFS (hazard ratio [HR], 1.040; 95% confidence interval [CI], 0.825-1.311; p=0.742) or OS (HR, 1.313; 95% CI, 0.874-1.971; p=0.190).
Conclusions:
Patients with LCMM have a higher CR rate after auto-HCT, but their PFS and OS were similar to patients with IgG/IgA MM.
Patient Characteristics
| Variables, No. (%)/median (range) . | IgG/IgA myeloma N= 606 . | Light chain myeloma N= 136 . | P . | |
|---|---|---|---|---|
| Median age at transplant, (y) | 59 (31-80) | 56 (32-78) | .004 | |
| Age >65 years | 138 (23) | 23 (17) | .134 | |
| Male | 357 (59) | 74 (54) | .337 | |
| Ethnicity | .731 | |||
| Caucasian | 399 (66) | 94 (69) | ||
| African American | 99 (16) | 22 (16) | ||
| Mixed | 87 (14) | 18 (13) | ||
| Asian | 16 (3) | 2 (2) | ||
| Cytogenetic abnormalities at diagnosis by conventional cytogenetics | ||||
| Diploid | 180 (30) | 36 (27) | .159 | |
| Hyperdiploid | 93 (15) | 9 (7) | .008 | |
| Hypodiploid | 27 (5) | 11 (8) | .082 | |
| t(11;14) | 4 (1) | 3 (2) | .092 | |
| Monosomy 13 / del 13 | 44 (7) | 9 (7) | .789 | |
| Other high-risk abnormalities | 2 (0) | 1 (1) | .456 | |
| Induction chemotherapy | ||||
| Bortezomib or IMiD-based | 507 (84) | 123 (90) | .046 | |
| Pre-transplant evaluation | ||||
| Bone marrow plasma cell, (%) | 2 (0-71) | 2 (0-50) | .136 | |
| Bone marrow plasma cell >10% | 90 (15) | 18 (13) | .735 | |
| Hemoglobin, (g/dL) | 11.3 (4.4-16.0) | 10.8 (6.8-15.3) | .025 | |
| Lactate dehydrogenase, (IL/L) | 526 (221-5062) | 526 (239-2748) | .522 | |
| Calcium, (mg/dL) | 9.0 (7.6-10.4) | 9.0 (7.5-11.0) | .055 | |
| Creatinine, (mg/dL) | 0.9 (0.4-12.5) | 0.9 (0.5-9.8) | .017 | |
| Beta-2 microglobulin (mg/dL) | 2.4 (1.1-40.0) | 2.8 (1.2-33.8) | .001 | |
| Time from diagnosis to auto-HCT (month) | 8.0 (1.9-174.4) | 6.8 (2.4-44.6) | .001 | |
| Pre-transplant disease status | .004 | |||
| ≥ CR | 24 (4) | 15 (11) | ||
| VGPR/PR | 545 (90) | 109 (80) | ||
| SD/PD | 37 (6) | 12 (9) | ||
| Conditioning regimen | .008 | |||
| Melphalan alone | 508 (84) | 126 (93) | ||
| Melphalan-based regimen | 98 (16) | 10 (7) | ||
| Final response after transplant | .080 | |||
| ≥ CR | 168 (28) | 52 (38) | ||
| VGPR/PR | 353 (58) | 70 (52) | ||
| SD/PD | 81 (13) | 14 (10) | ||
| Variables, No. (%)/median (range) . | IgG/IgA myeloma N= 606 . | Light chain myeloma N= 136 . | P . | |
|---|---|---|---|---|
| Median age at transplant, (y) | 59 (31-80) | 56 (32-78) | .004 | |
| Age >65 years | 138 (23) | 23 (17) | .134 | |
| Male | 357 (59) | 74 (54) | .337 | |
| Ethnicity | .731 | |||
| Caucasian | 399 (66) | 94 (69) | ||
| African American | 99 (16) | 22 (16) | ||
| Mixed | 87 (14) | 18 (13) | ||
| Asian | 16 (3) | 2 (2) | ||
| Cytogenetic abnormalities at diagnosis by conventional cytogenetics | ||||
| Diploid | 180 (30) | 36 (27) | .159 | |
| Hyperdiploid | 93 (15) | 9 (7) | .008 | |
| Hypodiploid | 27 (5) | 11 (8) | .082 | |
| t(11;14) | 4 (1) | 3 (2) | .092 | |
| Monosomy 13 / del 13 | 44 (7) | 9 (7) | .789 | |
| Other high-risk abnormalities | 2 (0) | 1 (1) | .456 | |
| Induction chemotherapy | ||||
| Bortezomib or IMiD-based | 507 (84) | 123 (90) | .046 | |
| Pre-transplant evaluation | ||||
| Bone marrow plasma cell, (%) | 2 (0-71) | 2 (0-50) | .136 | |
| Bone marrow plasma cell >10% | 90 (15) | 18 (13) | .735 | |
| Hemoglobin, (g/dL) | 11.3 (4.4-16.0) | 10.8 (6.8-15.3) | .025 | |
| Lactate dehydrogenase, (IL/L) | 526 (221-5062) | 526 (239-2748) | .522 | |
| Calcium, (mg/dL) | 9.0 (7.6-10.4) | 9.0 (7.5-11.0) | .055 | |
| Creatinine, (mg/dL) | 0.9 (0.4-12.5) | 0.9 (0.5-9.8) | .017 | |
| Beta-2 microglobulin (mg/dL) | 2.4 (1.1-40.0) | 2.8 (1.2-33.8) | .001 | |
| Time from diagnosis to auto-HCT (month) | 8.0 (1.9-174.4) | 6.8 (2.4-44.6) | .001 | |
| Pre-transplant disease status | .004 | |||
| ≥ CR | 24 (4) | 15 (11) | ||
| VGPR/PR | 545 (90) | 109 (80) | ||
| SD/PD | 37 (6) | 12 (9) | ||
| Conditioning regimen | .008 | |||
| Melphalan alone | 508 (84) | 126 (93) | ||
| Melphalan-based regimen | 98 (16) | 10 (7) | ||
| Final response after transplant | .080 | |||
| ≥ CR | 168 (28) | 52 (38) | ||
| VGPR/PR | 353 (58) | 70 (52) | ||
| SD/PD | 81 (13) | 14 (10) | ||
a) Progression-free survival, b) Overall survival in patients with light chain myeloma compared to those with IgG/IgA myeloma
a) Progression-free survival, b) Overall survival in patients with light chain myeloma compared to those with IgG/IgA myeloma
Shah:Celgene: Consultancy, Research Funding. Thomas:Novartis, Celgene, Acerta Pharmaceuticals, Idera Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal