Abstract
Patients with steroid-refractory acute graft versus host disease (aGVHD) have dismal outcomes. Historically, anti-thymocyte globulin has been used in this setting with prior reports demonstrating that even when patients respond, long-term survival occurs in only 5% of patients (Arai et al, 2012). Unfortunately, no therapy has been shown to improve outcomes for this high-risk group. Pentostatin, a potent adenosine deaminase inhibitor, was previously tested in a phase 1/ 2 study in steroid-refractory aGVHD and demonstrated an overall survival (OS) of 25% with a median follow-up of < 3 months (m). We performed a retrospective review of patients receiving pentostatin for steroid-refractory aGVHD with the goal of characterizing long-term outcomes.
Methods: All patients transplanted at MD Anderson Cancer Center from January, 2006 to December, 2014 who received at least one dose of pentostatin for steroid-refractory aGVHD were included in this analysis. Pentostatin was dosed at 1.5 mg/m2 on days 1-3 and repeated every two weeks as indicated. Patients who received the drug as GVHD prophylaxis, upfront therapy, beyond third-line, for classic chronic GVHD or following relapse were excluded. Primary endpoints included day 28 response and OS.
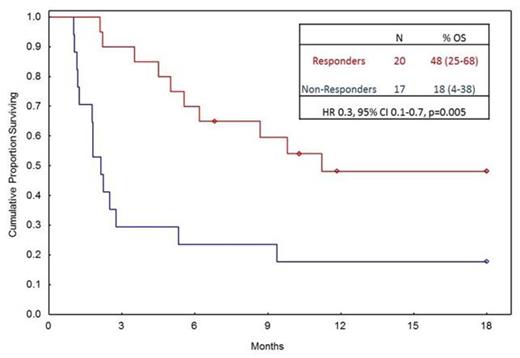
Results: A total of 60 patients received pentostatin as second (n=22) or third line (n=38) treatment for steroid-refractory aGVHD. The median age was 52 years (range, 2-70). First dose of pentostatin was administered at a median 69 days post-transplant (27-595) with a median of 3 doses provided (range, 1-9). A majority of patients had steroid-refractory lower GI (78%) and/or liver (43%) GVHD. Baseline characteristics described in table 1. The median time from initiation of steroids to pentostatin was 15 days (range, 4-172). OS at 18 m after pentostatin initiation was 21%, with median follow-up of 19 m (range, 7-77).A total of 22 (37%) patients died before day 28 and were considered non-responders. Day 28 response rate was 33% with 20 patients achieving a complete (n=11) or partial response (n=9). Pentostatin administration <10 days following initiation of steroid was the only predictor for day 28 response (HR 2.2, 95% CI 1.2-4.3, p=0.02). The median survival was 25 and 341 days in non-responders and responders, respectively. Landmark analysis starting on day 29 after pentostatin is shown in figure 1. Predictors for OS on multivariate analysis at 18 m included: day 28 complete/partial response (CR/PR) as a time dependent variable (HR 0.3, 95% CI 0.1-0.7, p=0.005), liver GVHD (HR 2.2, 95% CI 1.2-4.1, p=0.007), and age >60 (HR 1.9, 95% CI 0.99-3.6, p=0.05). Within our analysis, patients destined for early mortality within 30 days of pentostatin exhibited all these features: liver GVHD, receipt of pentostatin >100 days from transplant, and age >60, with an OS of 35% at 30 days compared to 72% in the absence of these features (p=0.001).
Conclusions: Patients with steroid refractory aGVHD have dismal outcomes. However, long-term survival does occur, especially when additional therapy is administered promptly. Novel strategies to identify patients who are destined to fail upfront therapies are likely to improve outcomes. We identified that earlier dosing of pentostatin after recognition of steroid refractoriness improved response rates. Further, patients without liver GVHD and who were ≤60 had better OS, identifying those who will benefit most from this therapy. Conversely, pentostatin should be avoided in patients >60 with liver GVHD as these patients had extremely high mortality with two-thirds dying by day 30.
Baseline characteristics at pentostatin initiation.
| Variable . | Patients (N=60) . |
|---|---|
| Preparative Regimen Intensity | |
| Non-myeloablative (%) | 22 (37) |
| Ablative (%) | 38 (63) |
| Overall GVHD Grade | |
| 2 (%) | 11 (18) |
| 3 (%) | 21 (35) |
| 4 (%) | 28 (47) |
| Skin GVHD Stage | |
| 0 (%) | 48 (80) |
| 1 (%) | 4 (7) |
| 2 (%) | 5 (8) |
| 3 (%) | 3 (5) |
| Lower GI GVHD Stage | |
| 0 (%) | 12 (20) |
| 1 (%) | 10 (17) |
| 2 (%) | 6 (10) |
| 3 (%) | 11 (18) |
| 4 (%) | 20 (33) |
| Unknown (%) | 1 (2) |
| Liver GVHD Stage | |
| 0 (%) | 33 (55) |
| 1 (%) | 4 (7) |
| 2 (%) | 9 (15) |
| 3 (%) | 6 (10) |
| 4 (%) | 7 (11) |
| Unknown (%) | 1 (2) |
| Number of GVHD sites | |
| 1 (%) | 40 (67) |
| 2 (%) | 15 (25) |
| ≥3 (%) | 5 (8) |
| Day 28 Response | |
| CR (%) | 11 (18) |
| PR (%) | 9 (15) |
| No Response (%) | 11 (18) |
| GVHD Progression (%) | 5 (9) |
| Died by Day 28 (%) | 22 (37) |
| Progression of Malignancy (%) | 2 (3) |
| Variable . | Patients (N=60) . |
|---|---|
| Preparative Regimen Intensity | |
| Non-myeloablative (%) | 22 (37) |
| Ablative (%) | 38 (63) |
| Overall GVHD Grade | |
| 2 (%) | 11 (18) |
| 3 (%) | 21 (35) |
| 4 (%) | 28 (47) |
| Skin GVHD Stage | |
| 0 (%) | 48 (80) |
| 1 (%) | 4 (7) |
| 2 (%) | 5 (8) |
| 3 (%) | 3 (5) |
| Lower GI GVHD Stage | |
| 0 (%) | 12 (20) |
| 1 (%) | 10 (17) |
| 2 (%) | 6 (10) |
| 3 (%) | 11 (18) |
| 4 (%) | 20 (33) |
| Unknown (%) | 1 (2) |
| Liver GVHD Stage | |
| 0 (%) | 33 (55) |
| 1 (%) | 4 (7) |
| 2 (%) | 9 (15) |
| 3 (%) | 6 (10) |
| 4 (%) | 7 (11) |
| Unknown (%) | 1 (2) |
| Number of GVHD sites | |
| 1 (%) | 40 (67) |
| 2 (%) | 15 (25) |
| ≥3 (%) | 5 (8) |
| Day 28 Response | |
| CR (%) | 11 (18) |
| PR (%) | 9 (15) |
| No Response (%) | 11 (18) |
| GVHD Progression (%) | 5 (9) |
| Died by Day 28 (%) | 22 (37) |
| Progression of Malignancy (%) | 2 (3) |
OS, starting at day 29, stratified by response.
Alousi:Therakos, Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal