Abstract
Introduction
Hepatic veno-occlusive disease, also called sinusoidal obstruction syndrome (VOD/SOS), is an unpredictable, potentially life-threatening complication of conditioning regimens for hematopoietic stem cell transplant (HSCT). Although VOD/SOS usually is thought of as a complication of HSCT, there is also a known risk in patients following chemotherapy in a non-HSCT setting. Severe hepatic VOD/SOS (ie, with multi-organ dysfunction [MOD]), may be associated with >80% mortality. Endothelial cell (EC) damage is a critical factor in the pathophysiology of VOD/SOS. Preclinical data suggest that defibrotide stabilizes ECs with direct, as well as EC-mediated, restoration of the thrombo-fibrinolytic balance. Defibrotide is approved for treatment of severe hepatic VOD/SOS in adult and pediatric patients in the European Union. In the United States, defibrotide is available as an investigational drug through an ongoing expanded-access protocol for treatment of hepatic VOD/SOS.
Methods
In the original protocol, patients were eligible if they had hepatic VOD/SOS by Baltimore criteria post-HSCT and MOD, defined by renal (tripling of creatinine levels or reduced creatinine clearance with or without dialysis) and/or pulmonary (need for oxygen supplementation with or without assisted ventilation) dysfunction. Symptoms of VOD/SOS were not considered adverse events (AEs) unless the event was considered serious. The protocol was later amended to include (1) post-chemotherapy patients with hepatic VOD/SOS; (2) patients with hepatic VOD/SOS without MOD, and (3) VOD/SOS per modified Seattle criteria. Enrolled patients received defibrotide 25 mg/kg/d in 4 divided doses for a recommended ≥21 days. Here, we describe efficacy and safety results with defibrotide for the subset of patients that developed VOD/SOS post-chemotherapy.
Results
Out of 642 patients who developed VOD/SOS and received ≥1 dose of defibrotide, 69 patients received chemotherapy without HSCT; 52% (n=36) had MOD, and 48% (n=33) did not. Median age was 8 years (range, <1 month-58.0 years), and 55 patients (80%) were ≤16 years (39 patients were children aged 2-11); 54% of patients were male. The most common primary diseases were acute lymphocytic leukemia (44%) and acute myelogenous leukemia (10%). Chemotherapeutic agents received by more than 30% of patients were vincristine, cyclophosphamide, cytarabine, doxorubicin, methotrexate and PEG-L-asparaginase. Antibody-drug conjugates linked to ozogamicin that are associated with development of VOD/SOS (gemtuzumab and inotuzumab) were received by 3 and 1 patient, respectively.
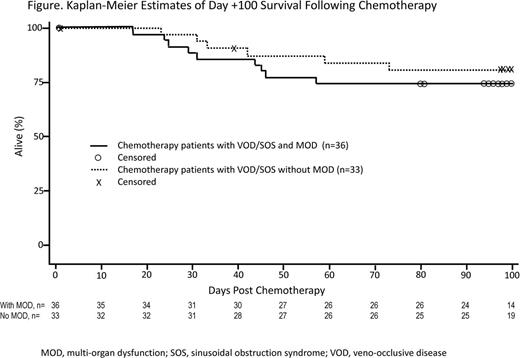
The Kaplan-Meier estimated day +100 survival rate was 77.4% (95% confidence interval, 65.4%-85.7%). For patients with MOD and without MOD, the Kaplan-Meier estimated day +100 survival rates were 74.3% (56.4%-85.7%) and 80.9% (62.3%-90.9%), respectively.
Overall, ≥1 AE was reported in 44 chemotherapy patients (63.8%). Of these, 14 (20.3%) had AEs assessed by the investigator as possibly, probably, or definitely related to defibrotide. Treatment-related AEs occurring in ≥2 patients were hypotension (4.3%), nausea (2.9%), vomiting (2.9%), and epistaxis (2.9%). Hemorrhagic AEs of any severity occurring in ≥2 patients were pulmonary (7.2%), epistaxis (5.8%), and gastric (2.9%). Serious AEs were reported in 26 patients (37.7%), most commonly multi-organ failure (7.2%), hypoxia (5.8%), and pulmonary hemorrhage (5.8%). AEs led to discontinuation in 4 patients (gastric, gastrointestinal and mouth hemorrhages, epistaxis, and hypotension). No treatment-related deaths were reported.
Conclusions
Day+100 survival of 77.4% in patients developing VOD/SOS following a variety of chemotherapy regimens without HSCT (80% of which were pediatric, primarily children) is a clinically encouraging finding. Defibrotide treatment in this group of 69 patients developing VOD/SOS post-chemotherapy was generally well-tolerated, with only 5.8% of patients discontinuing due to an AE and no treatment-related fatalities. Enrollment to the study continues.
Support: Jazz Pharmaceuticals.
Kernan:Gentium S.p.A.: Research Funding. Off Label Use: Defibrotide is an investigational treatment for hepatic veno-occlusive disease/sinusoidal obstruction syndrome in the United States.. Richardson:Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gentium S.p.A.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Grupp:Novartis: Consultancy, Research Funding. Antin:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Gentium S.p.A.: Membership on an entity's Board of Directors or advisory committees. Liang:Jazz Pharmaceuticals: Employment, Equity Ownership. Hume:Jazz Pharmaceuticals: Employment, Equity Ownership. Hannah:Jazz Pharmaceuticals: Consultancy. Nejadnik:Jazz Pharmaceuticals: Employment, Equity Ownership. Soiffer:Gentium SpA/Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal