Abstract
Allogeneic haematopoietic-stem-cell transplantation (HSCT) is the treatment of choice for many malignant and non-malignant disorders. However, Graft-versus -Host Disease (GvHD), the major complication of allogeneic HSCT, limits its wider application. Among different sites that can be involved, gastrointestinal GvHD represents the major cause of patients morbidity and mortality.
The infiltration of different cell subsets into target organs is an important step in GvHD pathogenesis, and the modulation of cell trafficking could represent a promising strategy for GvHD prophylaxis and treatment. Chemerin has been recently identified as a chemotactic protein, which is produced by several tissues during inflammation and binds the G protein-coupled receptor ChemR23, expressed by immature myeloid and plasmacytoid Dendritic Cells, macrophages and natural killer cells.
The aim of this study was to evaluate the potential role of Chemerin/ChemR23 axis in the pathogenesis of GvHD, in order to identify disease-specific pathways exploitable for developing new potential therapeutic targets. For this purpose, lethally irradiated Balb/C recipient mice were transplanted with bone marrow cells and splenocytes obtained from ChemR23deficient (ChemR23-/-) C57BL6 mice. After transplantation, mice were monitored daily for survival and GvHD severity. Recipient mice were sacrificed at different time points to evaluate Chemerin production and leukocytes infiltration in skin, lung, liver, and gut by using different techniques, such as ELISA, histopathology, FACS and PCR.
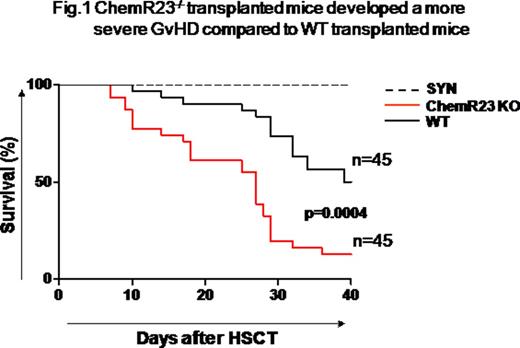
Starting from day +6 after transplantation, Chemerin plasma levels appeared significantly higher in both wild type (WT) and ChemR23-/- mice who developed GvHD (WT mean level=86.8 ng/ml; range=80.1-91.9; ChemR23-/- mean level=83.8 ng/ml; range=70.5-103.6, n=6, day+7) compared to syngeneic controls (mean level=62.8 ng/ml; range=49.2-82.5, n=6, day+7), p=0.02. Interestingly, ChemR23-/- mice developed a more severe GvHD compared to mice transplanted with WT cells. In particular, ChemR23-/- transplantedmice showed a higher mortality rate (at day +28 after HSCT: 85% mortality in ChemR23-/- vs 25% in WT, p=0.0004, n=45) (Fig. 1). Differences in GvHD score between ChemR23-/- and WT transplanted mice resulted by a significantly increase in weight loss, associated to severe diarrhea. In accordance, histopathologic analysis performed on GvHD target organs showed a significantly higher GvHD score in large intestine of ChemR23-/- transplanted mice, whereas no differences were found in other GvHD target organs. In addition, a deeper histological analysis on large intestine showed that tissue damage is characterized by crypt hyperplasia and atrophy, epithelium apoptosis and colitis. Moreover, FACS analysis of large intestine infiltrating leukocytes showed an higher neutrophils frequency in ChemR23-/- transplantedmice compared to WT (neutrophils=10.39%, range 6.5%-15.1% versus neutrophils=5.84%, range 2.6%-12.8%, respectively, p=0.001, n=10). This observation was also obtained by analyzing the mesenteric lymphnode. The higher neutrophils infiltration was also confirmed by immunohistochemistry evaluating the number of myeloperoxidase (MPO) positive cells and by quantitative PCR detecting MPO expression (ChemR23-/- mean 2-ΔΔCt =22.35, range 2.43-46.05; WT mean 2-ΔΔCt =6.42, range 0.20-19.23; p=0.04, n=8).
All these findings suggest that the Chemerin/ChemR23 axis play a crucial role in intestinal GVHD. Further studies are needed to better understand the mechanisms underling the severe damage observed in the gastrointestinal tract of mice transplanted with ChemR23-/- cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal