Abstract
Although survival in AL patients is related in part to the number of marrow clonal plasma cells (PC) (1, 2), staging for AL patients is based on end-organ damage without a clear role for cytogenetic aberrations in clonal PC. Recently several groups have described potential roles for gain 1q and t(11;14) as prognostic markers in AL, and possibly as predictive of response to therapy (3-5). In multiple myeloma, del 17p is a cytogenetic aberration present in about 10% at diagnosis and its prognostic impact depends on the percentage of clonal PC with del 17p (6). The relevance of del 17p in AL is undefined. We report our initial analysis of the outcomes and survival of the first multinational retrospective study of AL patients with del 17p as a feature of their clonal plasma cell disease and include preliminary findings from an age-based case-control analysis.
AL patients with del 17p were identified and their clinical characteristics and outcomes summarized. Methods for determining the presence of del 17p were reviewed. We compared the overall survival (OS) of patients in whom del 17p was identified in < or ≥ 50% of clonal cells, and evaluated the impact of bortezomib-based therapy and cardiac stage on OS in newly diagnosed and relapsed patients. These cases were then matched for age (± 5 years) with a control cohort from the Amyloidosis Treatment and Research Center in Pavia, IT, representing a subset of patients reported previously (7). Differences between cases and controls for OS were evaluated by Kaplan-Meier with statistical significance by the log-rank test. P < 0.05 was the threshold for significance.
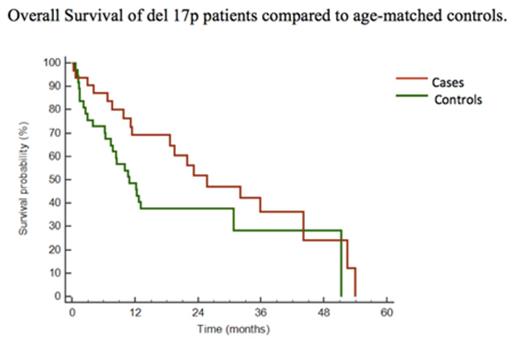
Thirty-four AL patients with del 17p in clonal cells were identified, 30 at diagnosis and 4 at relapse. Thirty-one cases had del 17p assessed on CD138-selected marrow cells and 3 on marrow mononuclear cells; for the latter, the percentage of marrow PC was used to estimate the del 17p fraction. Commercially available methods were used at all centers. Median age was 66 years and males constituted 53% of cases. Cardiac involvement was present in 72% of cases, 41% of whom had stage III involvement. Ninety-four percent had free light-chain (FLC) clones of lambda isotype with a median difference between involved and uninvolved FLC (dFLC) of 249mg/L and a median percentage of marrow PC of 18%. In all 34 cases the median percentage of clonal PC with del 17p by FISH was 42% (range 2-93%) while in newly diagnosed AL patients it was 37%. Eighty-three percent of cases had del 17p in combination with other cytogenetic abnormalities including t(11;14) in 36% and gain 1q in 18%. Among the newly diagnosed cases, 32% had ≥ 50% of clonal cells with del 17p and had a median OS of 23.5 months while those with < 50% survived a median of 33 months (P = 0.12). Seventy-three percent of patients responded to initial therapy and 32% had ≥ VGPR. Twenty-three percent had a cardiac response to initial therapy. There was no difference in OS between patients who had bortezomib-based therapy and those who did not, and no difference between the OS of cardiac stage 1/2 and stage 3 patients. One patient with 37% del 17p and a complex karyotype at baseline progressed at relapse to both advanced AL cardiac involvement and plasma cell leukemia with over 90% del 17p containing PC, suggesting that del 17p may confer risk in AL as in MM under certain circumstances. In the preliminary case-control analysis, OS was no different between cases and controls (Fig 1, P = 0.32).
Recent data indicate that the more common cytogenetic abnormalities in AL, namely t(11;14) and gain 1q, are associated with differences in response to melphalan or bortezomib. In this series of AL cases with del 17p, we find suggestive evidence that high levels of clonal cells with del 17p may confer a poorer prognosis than already exists as a consequence of the tropism of AL light chains for the heart. We are seeking more cases for inclusion in this series and conducting both a multivariate analysis and a detailed case-control comparison which may shed light on this issue.
References
(1) Comenzo et al. Blood 2001;98(3):714-20.
(2) Warsame et al. Blood Cancer Journal 2015;5, e-310, e-pub 1 May 2015
(3) Bochtler et al. Amyloid. 2014 Mar;21(1):9-17.
(4) Bochtler et al. J Clin Oncol. 2015 Apr 20;33(12):1371-8.
(5) Zhou et al. Clin Lymphoma Myeloma Leuk 2012;12(1):49-58.
(6) An et al. Clin Cancer Res; 21(9); 2148-56.
(7) Palladini et al. Blood 2015, e-pub 18 May 2015
Hegenbart:Janssen: Honoraria. Landau:Prothena: Consultancy, Honoraria; Janssen: Consultancy; Spectrum Pharmaceuticals: Honoraria; Janssen: Consultancy; Onyx: Honoraria, Research Funding; Takeda: Research Funding. Avet-Loiseau:onyx: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; millenium: Membership on an entity's Board of Directors or advisory committees; onyx: Membership on an entity's Board of Directors or advisory committees; jansen: Membership on an entity's Board of Directors or advisory committees; jansen: Membership on an entity's Board of Directors or advisory committees; millenium: Membership on an entity's Board of Directors or advisory committees; celgene: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Hansen:Celgene GmbH: Honoraria. Schönland:Janssen, Prothena: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Comenzo:Janssen: Research Funding; Prothena: Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees; Takeda Millennium: Membership on an entity's Board of Directors or advisory committees; Takeda Millennium: Research Funding; Karyopharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal